Cobalt ii chloride molar mass
Cobalt Ii Chloride Molar Mass. Claims of the formation of tri- and tetrahydrates have not been confirmed. The anhydrous without water blue form can be made by reacting cobalt with chlorine. Following a single oral dose of cobalt chloride the blood cobalt concentration-time curve in male fischer 344 rats was triphasic peaked at 32 hr and had an absorptive half-life of 09 hr an elimination phase half-life of 39 hr and a terminal elimination half-life of 229 hr. Lets consider the saturated solution of silver chloride AgCl.
 Molar Mass Molecular Weight Of Cocl2 6h2o Youtube From youtube.com
Molar Mass Molecular Weight Of Cocl2 6h2o Youtube From youtube.com
Cu 2 Cl 2. The nickel chlorides are. CobaltII sulfide beta CoS. K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. CobaltII Nitrite CO2 Carbon Dioxide CoCl2 CobaltII Chloride COCl2 Phosgene CoSO4 CobaltII Sulfate CrOH3 Chromium Hydroxide Cr2O3 ChromiumIII Oxide CrCl3 ChromiumIII Chloride CS2 Carbon Disulfide CsCl Caesium Chloride CuCN2 CopperII Cyanide CuNO32 CopperII Nitrate CuOH2 CopperII Hydroxide Cu2O CopperI Oxide Cu2S Copper. The anhydrous salt is yellow but the more familiar hydrate NiCl 2 6H 2 O is green.
Cu 3 AsO 4 2.
Lets consider the saturated solution of silver chloride AgCl. Lets consider the saturated solution of silver chloride AgCl. Farbmarkierung colour-marks BioQuick Brackets FACE Evolution II Prescription OK Maxillary Tooth Torque Angulation InOut Rotation 12 8 3 3 0 0 0 0 -30 -30 5 9 8 8 0 0 0 0 0 0 10 13 08 08 09 09 09 09 10 distal 6 distal 1 Centrals 2 Laterals Cuspids 3 4 5 Cuspids hook Bicuspids Bicuspids. Heres an example to better demonstrate the concept. The domain magnetization of elemental iron or cobalt exceeds that of magnetite at normal temperatures by three to fourfold giving incentive to the preparation of more highly magnetizable ferrofluids containing these metals. CopperII iodate monohydrate.
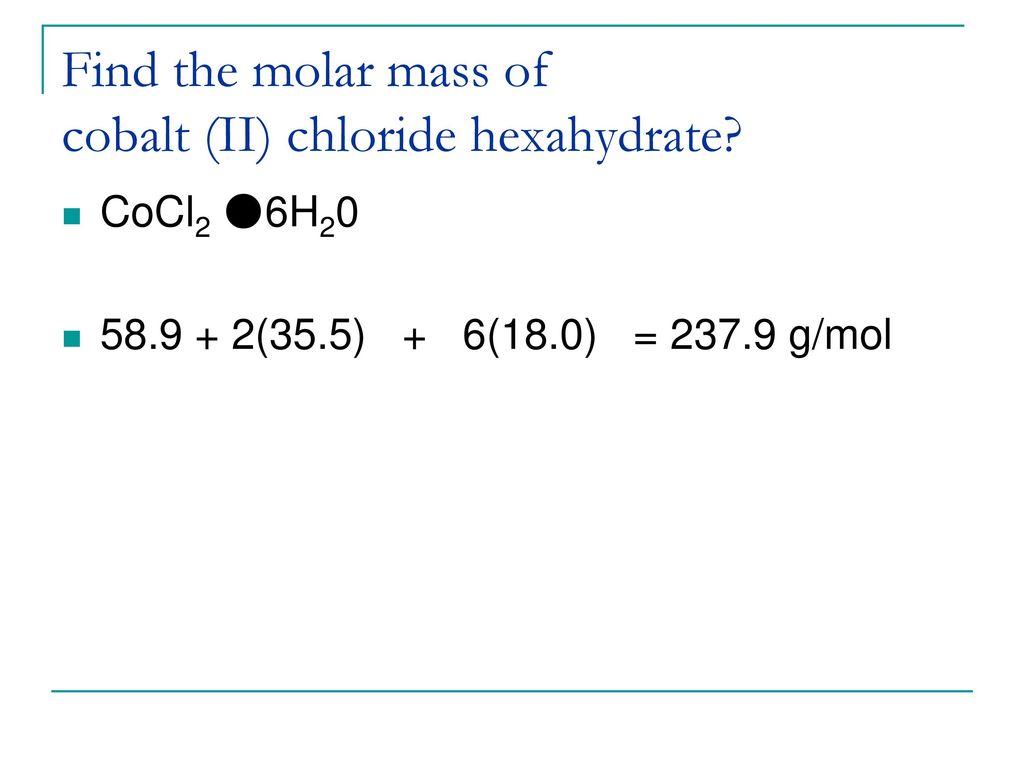 Source: slideplayer.com
Source: slideplayer.com
NickelII chloride in various forms is the most important source of nickel for chemical synthesis. The anhydrous without water blue form can be made by reacting cobalt with chlorine. A problem that arises is the reactivity of the elemental particles with atmospheric oxygen. NickelII chloride in various forms is the most important source of nickel for chemical synthesis. The domain magnetization of elemental iron or cobalt exceeds that of magnetite at normal temperatures by three to fourfold giving incentive to the preparation of more highly magnetizable ferrofluids containing these metals.
 Source: youtube.com
Source: youtube.com
The compound forms several hydrates CoCl 2 n H 2 O for n 1 2 6 and 9. CobaltII sulfide beta CoS. Lets consider the saturated solution of silver chloride AgCl. Following a single oral dose of cobalt chloride the blood cobalt concentration-time curve in male fischer 344 rats was triphasic peaked at 32 hr and had an absorptive half-life of 09 hr an elimination phase half-life of 39 hr and a terminal elimination half-life of 229 hr. Following intravenous administration 101 of the dose was excreted in the feces indicating that cobalt.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1993 prepared dispersions of iron-nitride particles for. 1295994 gmol anhydrous 23769 gmol hexahydrate Appearance yellow-brown crystals. Heres an example to better demonstrate the concept. NickelII chloride in various forms is the most important source of nickel for chemical synthesis. C 2 H 2 Cl 2 O 2.

NickelII chloride or just nickel chloride is the chemical compound NiCl 2. 1993 prepared dispersions of iron-nitride particles for. CoCl 2 2H 2 O — 1658686 g in one mole mass of two moles of water — 360296 g decimal percent of water in the hydrate — 360296 g 1658686 g 0217218. It can be oxidized to cobaltIII compounds although cobaltIII chloride does not exist. Cobalt chloride can be used to test for chloride ions in this way.
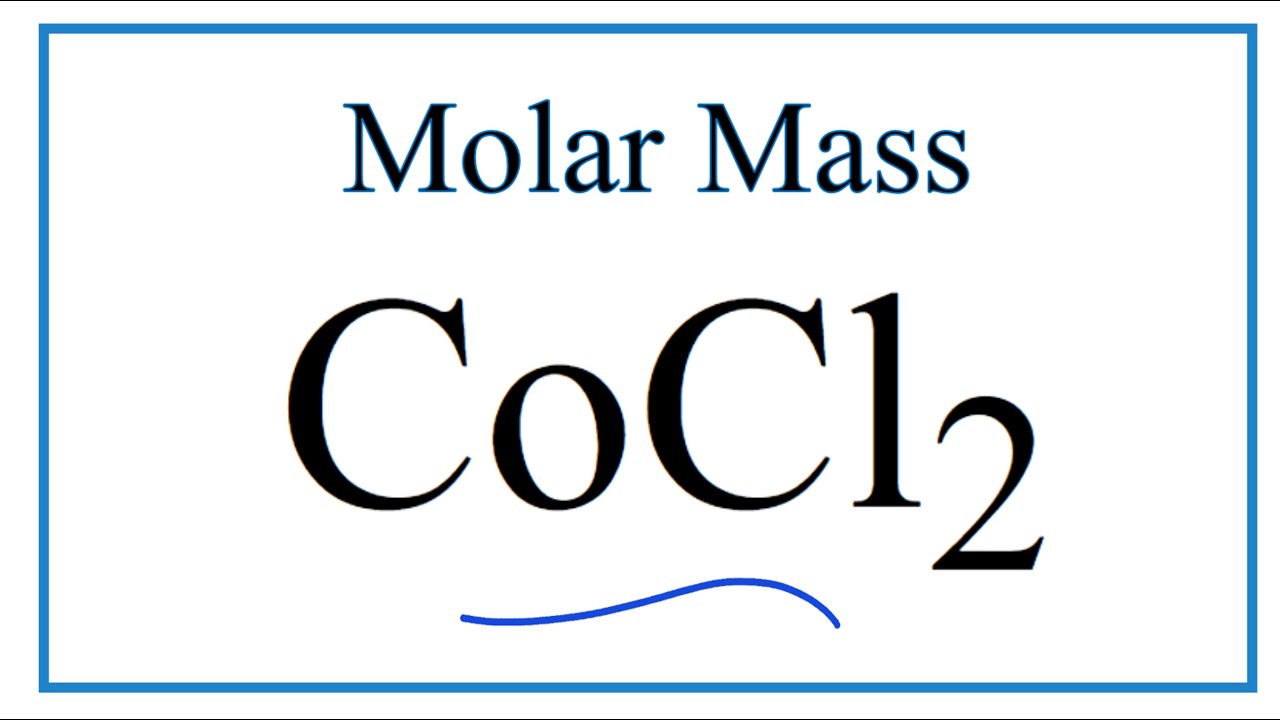 Source: youtube.com
Source: youtube.com
The combination of aqueous solutions of silver nitrate and sodium chloride is an easy method of synthesizing silver chloride. 1 Determine the percentage of water in cobaltII chloride dihydrate. It can also be formed by reacting with cobaltII chloride with silver nitrate. 1295994 gmol anhydrous 23769 gmol hexahydrate Appearance yellow-brown crystals. Diethyl ether C 2 H 5.
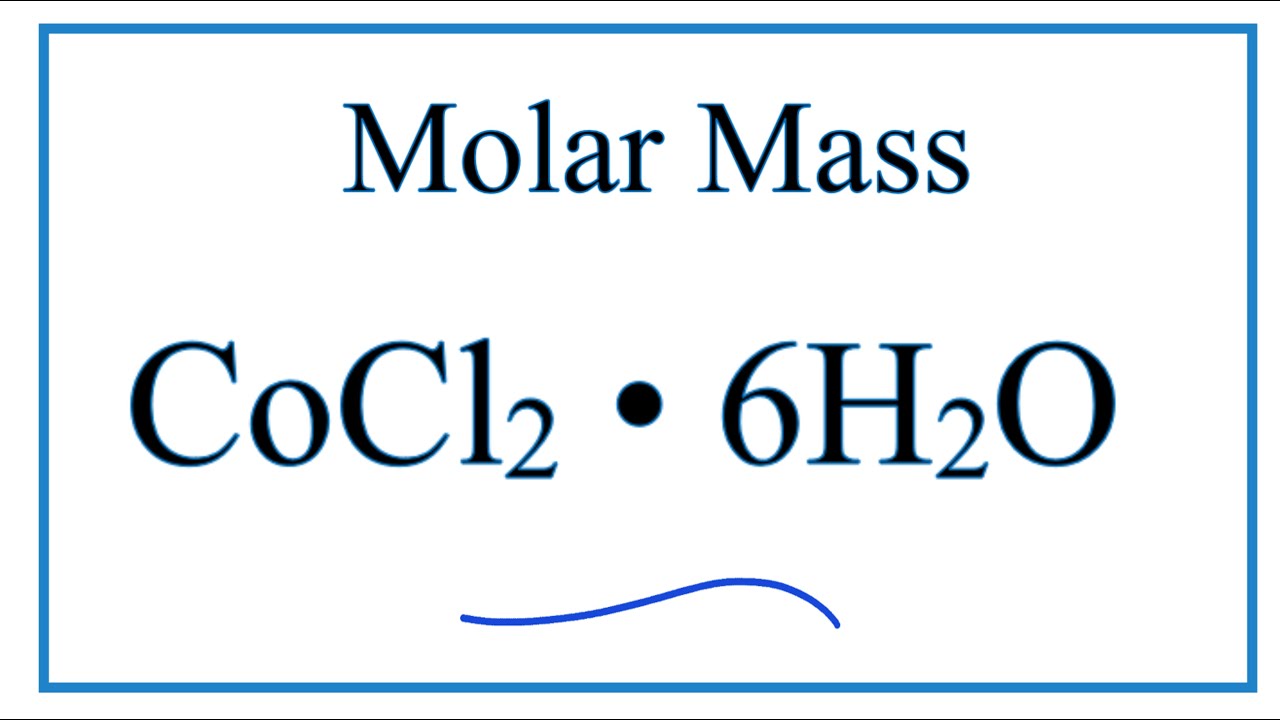 Source: youtube.com
Source: youtube.com
Cu 3 AsO 4 2. K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. A problem that arises is the reactivity of the elemental particles with atmospheric oxygen. It can also be formed by reacting with cobaltII chloride with silver nitrate. Chemical Reaction Formula Atomic Mass Formula Chemical Formula Enthalpy Formula Entropy Formula Molality Formula Molar Mass Formula Molarity Formula Structural Formula Molecular Formula Chemical Compound Formula Chemical Equilibrium Formula Normality Formula Photosynthesis Formula Grams to Moles Conversion Formula Moles to Grams Conversion Formula Radioactive Decay Formula.
The domain magnetization of elemental iron or cobalt exceeds that of magnetite at normal temperatures by three to fourfold giving incentive to the preparation of more highly magnetizable ferrofluids containing these metals. 2 Determine mass of water in 2413 g. It can be oxidized to cobaltIII compounds although cobaltIII chloride does not exist. CobaltII Nitrite CO2 Carbon Dioxide CoCl2 CobaltII Chloride COCl2 Phosgene CoSO4 CobaltII Sulfate CrOH3 Chromium Hydroxide Cr2O3 ChromiumIII Oxide CrCl3 ChromiumIII Chloride CS2 Carbon Disulfide CsCl Caesium Chloride CuCN2 CopperII Cyanide CuNO32 CopperII Nitrate CuOH2 CopperII Hydroxide Cu2O CopperI Oxide Cu2S Copper. CopperII iodate monohydrate.

K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. 1295994 gmol anhydrous 23769 gmol hexahydrate Appearance yellow-brown crystals. C 2 H 2 Cl 2 O 2. It can be oxidized to cobaltIII compounds although cobaltIII chloride does not exist. The combination of aqueous solutions of silver nitrate and sodium chloride is an easy method of synthesizing silver chloride.
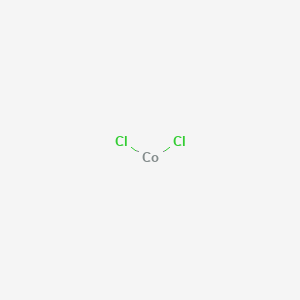
NickelII chloride or just nickel chloride is the chemical compound NiCl 2. It can also be formed by reacting with cobaltII chloride with silver nitrate. The domain magnetization of elemental iron or cobalt exceeds that of magnetite at normal temperatures by three to fourfold giving incentive to the preparation of more highly magnetizable ferrofluids containing these metals. K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. CobaltII sulfide beta CoS.
 Source: nagwa.com
Source: nagwa.com
K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. 1295994 gmol anhydrous 23769 gmol hexahydrate Appearance yellow-brown crystals. NickelII chloride in various forms is the most important source of nickel for chemical synthesis. The domain magnetization of elemental iron or cobalt exceeds that of magnetite at normal temperatures by three to fourfold giving incentive to the preparation of more highly magnetizable ferrofluids containing these metals. It can be oxidized to cobaltIII compounds although cobaltIII chloride does not exist.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title cobalt ii chloride molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






