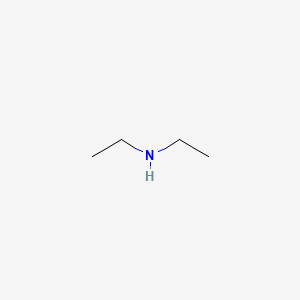
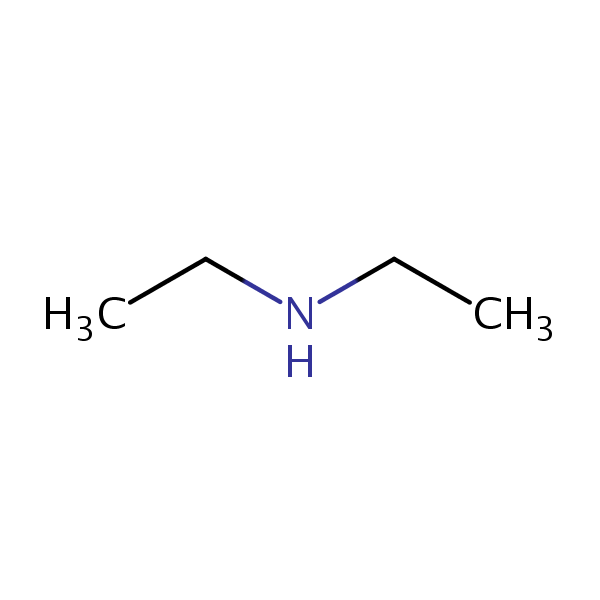
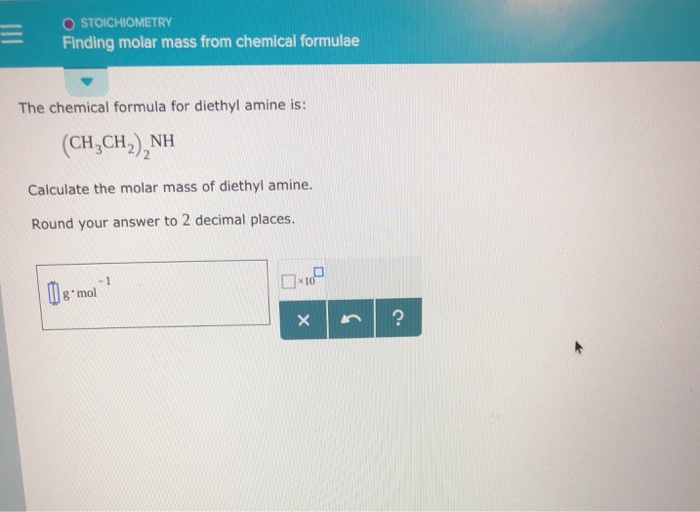
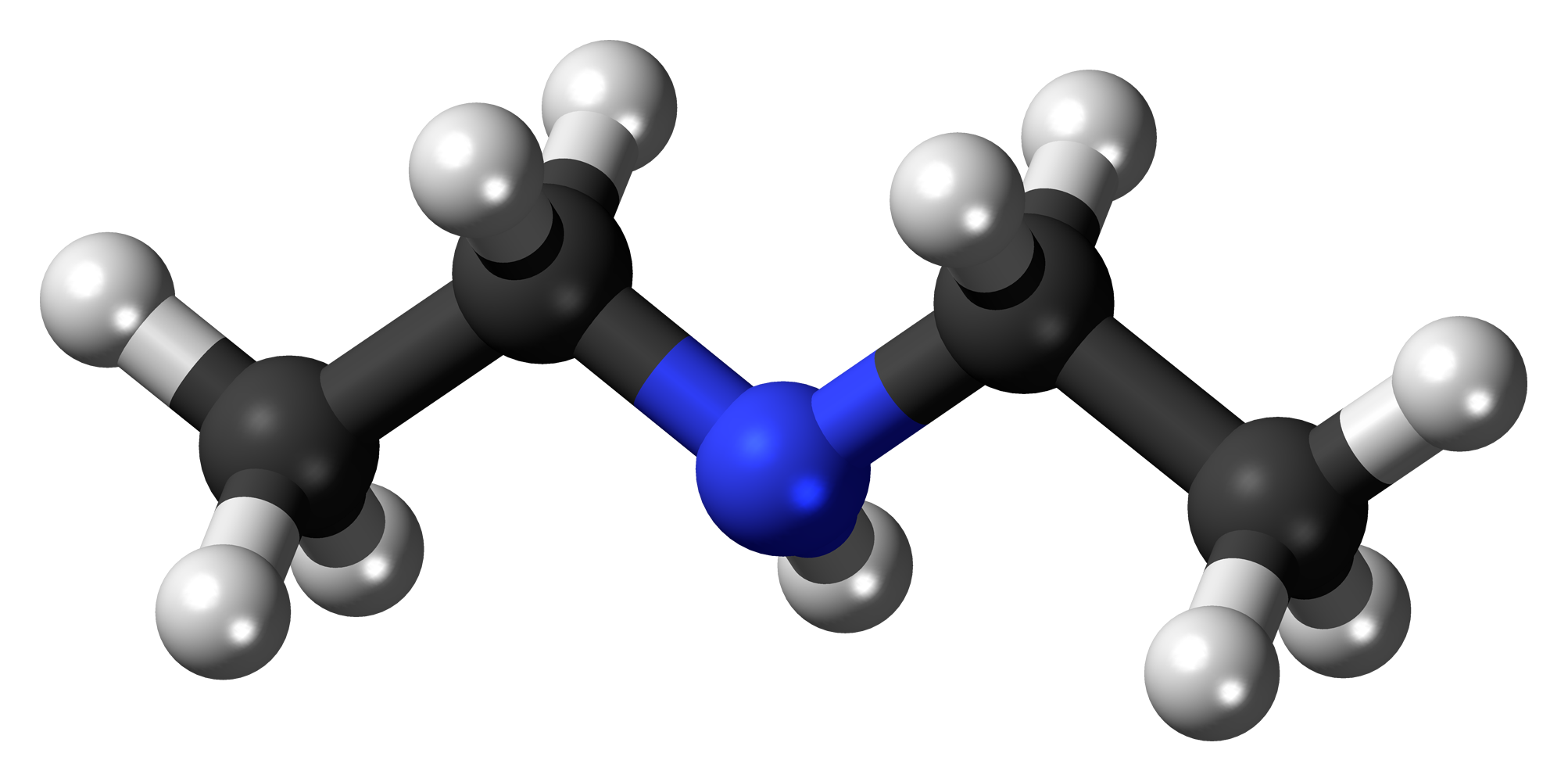
Diethylamine molar mass
Diethylamine Molar Mass. Nov 18 2001. Acidity pK a 1098 of ammonium. 22335 K Boiling point. Way to think outside the box though thats how we 14 butanediol.
 Diethylamine Molecular Weight C4h11n Over 100 Million Chemical Compounds Mol Instincts From molinstincts.com
Diethylamine Molecular Weight C4h11n Over 100 Million Chemical Compounds Mol Instincts From molinstincts.com
Naphthalenesulfonic acid-formaldehyde condensate sodium salt. The TA will add your diethyl amine for you from the reagent bottle to minimize the stench in the lab. Silicoaluminophosphate molecular sieve SAPO-41 was synthesized with di-n-propylamine DPA and a mixture of DPA and diethylamine DEA as templates. For C20H25N3O the molar mass. 1305 to 1334 F. How many molar equivalents of diethyl amine are used in step 2.
How many molar equivalents of diethyl amine are used in step 2.
3279 to 3295 K Solubility in water. Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5. 242975 kPa Henrys law constant k H 150 μmol Pa 1 kg 1. How many molar equivalents of diethyl amine are used in step 2. Weight of ethanol. Nov 18 2001.
To verify this point we first calculate the PL peaks of CsPbI 3 based RPP QWs with different n values based on a model using the modified effective mass approximation. Acidity pK a 1098 of ammonium. Orbital theory shows that there should exist a double bond single bond answers search this. It differs by the addition of a phenyl grouping to the GABA structure. α-Methylstyrene-vinyltoluene copolymer resins molar ratio 1α-methylstyrene to 3 vinyltoluene Mineral oil white.
 Source: sielc.com
Source: sielc.com
The TA will add your diethyl amine for you from the reagent bottle to minimize the stench in the lab. Three basic requirements must be met for explosion to take place. 1305 to 1334 F. Naphthalenesulfonic acid-formaldehyde condensate sodium salt. 73139 gmol 1 Appearance Colourless liquid Odor.
 Source: chegg.com
Source: chegg.com
Nov 18 2001. 548 to 564 C. 22335 K Boiling point. Oct 15 2001 oxidation of n-butane to maleic acid. Double bond between the two carbons in a C2 molecule with very low energy are core 1s orbitals core.
 Source: chegg.com
Source: chegg.com
It differs by the addition of a phenyl grouping to the GABA structure. The products were characterized by XRD XRF SEM MAS NMR and IR. Diclofenac sold under the brand name Voltaren among others is a nonsteroidal anti-inflammatory drug NSAID used to treat pain and inflammatory diseases such as gout. The butane acts as a solvent and separates the essential oils made up of cannabinoids terpenes trichomes and flavonoids from the cannabis plant. α-Methylstyrene-vinyltoluene copolymer resins molar ratio 1α-methylstyrene to 3 vinyltoluene Mineral oil white.
 Source: differencebetween.com
Source: differencebetween.com
Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N. Double bond between the two carbons in a C2 molecule with very low energy are core 1s orbitals core. 3279 to 3295 K Solubility in water. Mono- and bis-octadecyldiethylene oxide phosphates CAS Reg.
 Source: molinstincts.com
Source: molinstincts.com
3279 to 3295 K Solubility in water. Improvements in pain last for as much as eight hours. Oct 15 2001 oxidation of n-butane to maleic acid. α-Methylstyrene-vinyltoluene copolymer resins molar ratio 1α-methylstyrene to 3 vinyltoluene Mineral oil white. Acidity pK a 1098 of ammonium.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is taken by mouth rectally in a suppository used by injection or applied to the skin. Silicoaluminophosphate molecular sieve SAPO-41 was synthesized with di-n-propylamine DPA and a mixture of DPA and diethylamine DEA as templates. Naphthalenesulfonic acid-formaldehyde condensate sodium salt. Improvements in pain last for as much as eight hours. Nov 18 2001.

Double bond between the two carbons in a C2 molecule with very low energy are core 1s orbitals core. Weight of ethanol. Naphthalenesulfonic acid-formaldehyde condensate sodium salt. Compared with the single-template method the double-template method needs lower. How many molar equivalents of diethyl amine are used in step 2.
 Source: wikidata.org
Source: wikidata.org
Of amide 1 used record this mass. Oct 15 2001 oxidation of n-butane to maleic acid. Nov 18 2001. These data are consistent with the experimental values cited in the problem. 26 34 The results are shown in Table S2 Supporting Information.

Source of ignition - spark or high heat. Oct 15 2001 oxidation of n-butane to maleic acid. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N. 26 34 The results are shown in Table S2 Supporting Information. 3279 to 3295 K Solubility in water.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title diethylamine molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





