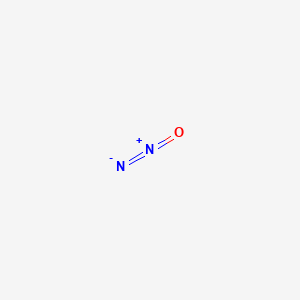
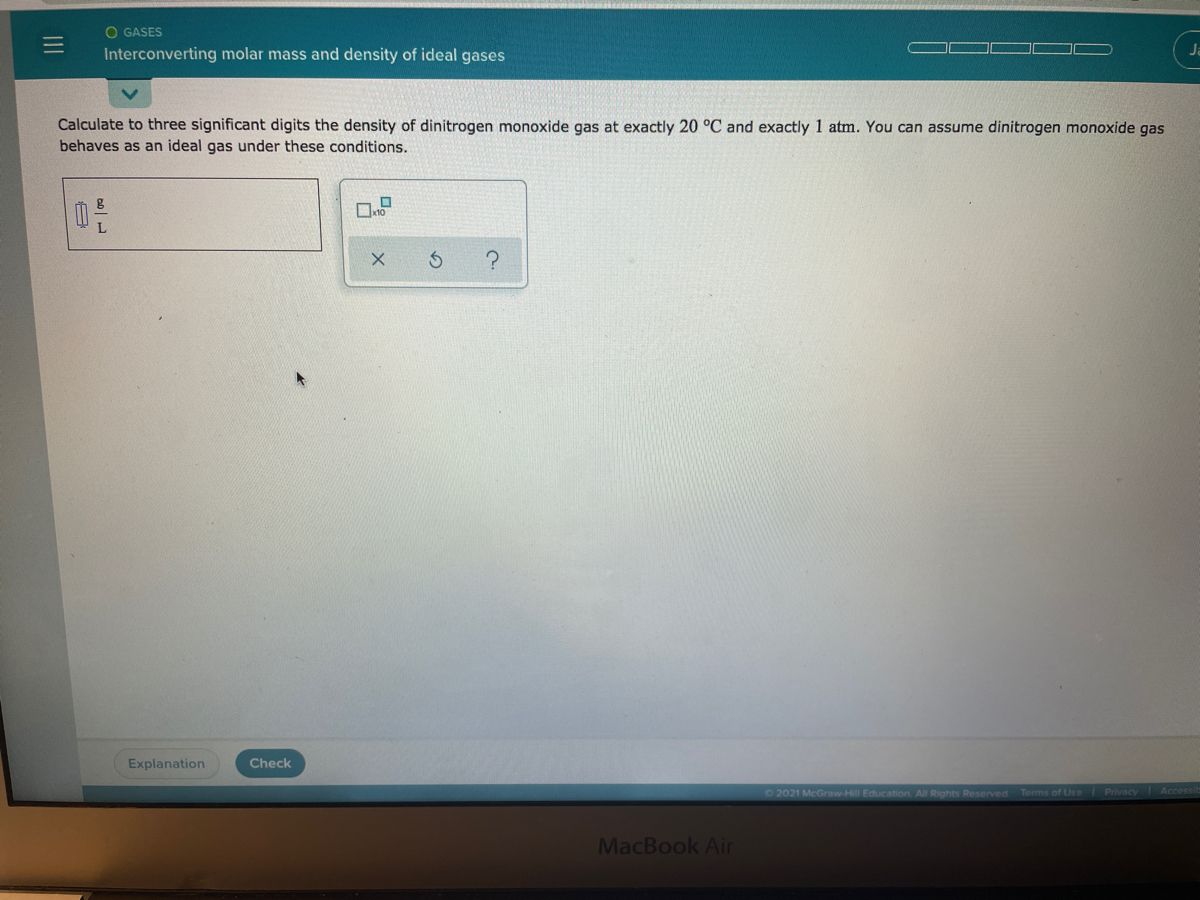
Dinitrogen monoxide gas molar mass
Dinitrogen Monoxide Gas Molar Mass. First the molar mass of ammonia is calculated to be 1704 gmol. What is the molar mass of the gas. How would you. It forms an equilibrium mixture with nitrogen dioxideIts molar mass is 92011 gmol.
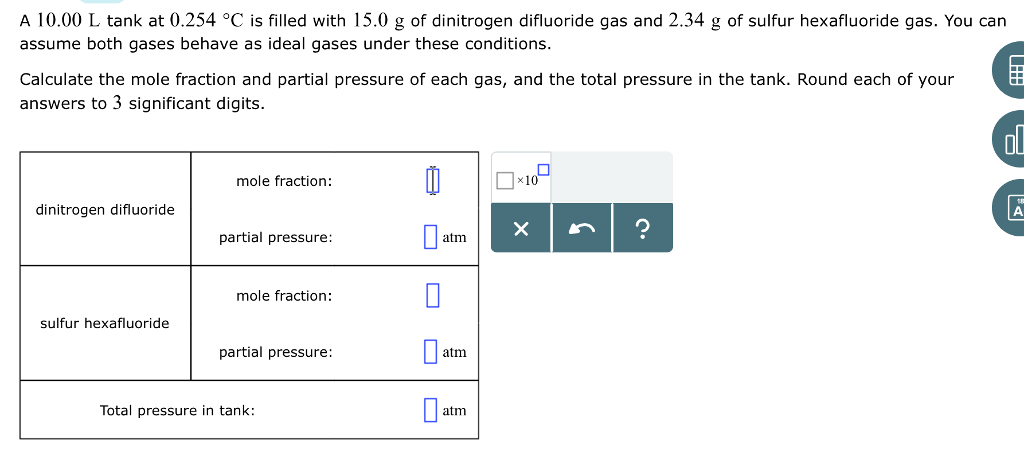
Nitrous oxide has significant medical uses especially in surgery and. How many grams of lithium metal are required to react with a 28g mass of dinitrogen gas. The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion or its reverse a mass-mole. Given that 66 moles of carbon monoxide gas are present in a 433L container what is the pressure of the gas in atm if the temperature is 84C. The representative particles can be atoms molecules or formula units of ionic compounds. Polyurethane Foam Structure C 27 H 36 N 2 O 10.
Nitrous oxide has significant medical uses especially in surgery and.
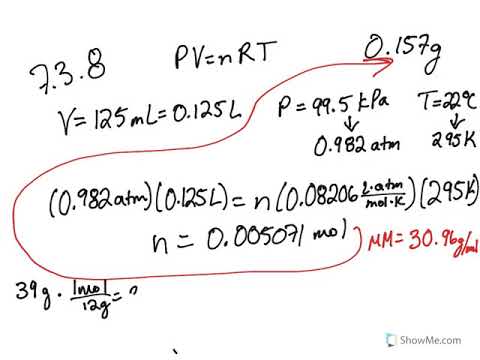
The ideal gas law can be used to find the density of a gas at conditions that are not standard. Calculating Density of a Gas. What is the molar mass of the gas. Molecular Weight Molar Mass. Given that 66 moles of carbon monoxide gas are present in a 433L container what is the pressure of the gas in atm if the temperature is 84C. If 230 g occupies 0870 L at 690 torr and 37 degrees C.
 Source: chemistrypage.in
Source: chemistrypage.in
It forms an equilibrium mixture with nitrogen dioxideIts molar mass is 92011 gmol. If 230 g occupies 0870 L at 690 torr and 37 degrees C. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. An experiment shows that a 245mL gas sample has a mass of 0436g at a pressure of 757 mmHg and a temperature of 29C. How would you.
 Source: chem.libretexts.org
Source: chem.libretexts.org
C 27 H 36 N 2 O 10. Dinitrogen tetroxide commonly referred to as nitrogen tetroxide NTO and occasionally usually among ex-USSRRussia rocket engineers as amyl is the chemical compound N 2 O 4It is a useful reagent in chemical synthesis. For example we will determine the density of ammonia gas NH 3 at 0913 atm and 20C assuming the ammonia is ideal. The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion or its reverse a mass-mole. It forms an equilibrium mixture with nitrogen dioxideIts molar mass is 92011 gmol.
 Source: youtube.com
Source: youtube.com
Given that 66 moles of carbon monoxide gas are present in a 433L container what is the pressure of the gas in atm if the temperature is 84C. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Ammonia is produced in the reaction 3H_2 N_2 - 2NH_3. The representative particles can be atoms molecules or formula units of ionic compounds. How would you.
 Source: clutchprep.com
Source: clutchprep.com
First the molar mass of ammonia is calculated to be 1704 gmol. How many liters of oxygen gas are. The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion or its reverse a mass-mole. To alleviate the limitations of mass transport flow cells with gas-diffusion electrodes GDEs have been developed and used to investigate electrochemical CO 2 or CO reduction 192021222324. First the molar mass of ammonia is calculated to be 1704 gmol.
Source: chemspider.com
The ideal gas law can be used to find the density of a gas at conditions that are not standard. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. Polyurethane Foam Structure C 27 H 36 N 2 O 10. If 230 g occupies 0870 L at 690 torr and 37 degrees C.

Physical Properties of Polyurethane Foam C 27 H 36 N 2 O 10. Physical Properties of Polyurethane Foam C 27 H 36 N 2 O 10. What mass of NH_3 could be produced if 125 H_2 reacts with excess nitrogen. Calculating Density of a Gas. The ideal gas law can be used to find the density of a gas at conditions that are not standard.
 Source: chegg.com
Source: chegg.com
C 27 H 36 N 2 O 10. This relationship is frequently used in the laboratory. Nitrous oxide commonly known as laughing gas nitrous or nos is a chemical compound an oxide of nitrogen with the formula N 2 OAt room temperature it is a colourless non-flammable gas with a slight metallic scent and tasteAt elevated temperatures nitrous oxide is a powerful oxidiser similar to molecular oxygen. Dinitrogen tetroxide commonly referred to as nitrogen tetroxide NTO and occasionally usually among ex-USSRRussia rocket engineers as amyl is the chemical compound N 2 O 4It is a useful reagent in chemical synthesis. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
 Source: toppr.com
Source: toppr.com
What is HClaq in the following scenario. Nitrous oxide commonly known as laughing gas nitrous or nos is a chemical compound an oxide of nitrogen with the formula N 2 OAt room temperature it is a colourless non-flammable gas with a slight metallic scent and tasteAt elevated temperatures nitrous oxide is a powerful oxidiser similar to molecular oxygen. The representative particles can be atoms molecules or formula units of ionic compounds. Solid with open cellular structure. Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts.
 Source: youtube.com
Source: youtube.com
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Calculating Density of a Gas. Physical Properties of Polyurethane Foam C 27 H 36 N 2 O 10. To alleviate the limitations of mass transport flow cells with gas-diffusion electrodes GDEs have been developed and used to investigate electrochemical CO 2 or CO reduction 192021222324. It forms an equilibrium mixture with nitrogen dioxideIts molar mass is 92011 gmol.
 Source: bartleby.com
Source: bartleby.com
Nitrous oxide commonly known as laughing gas nitrous or nos is a chemical compound an oxide of nitrogen with the formula N 2 OAt room temperature it is a colourless non-flammable gas with a slight metallic scent and tasteAt elevated temperatures nitrous oxide is a powerful oxidiser similar to molecular oxygen. Nitrous oxide has significant medical uses especially in surgery and. The representative particles can be atoms molecules or formula units of ionic compounds. It forms an equilibrium mixture with nitrogen dioxideIts molar mass is 92011 gmol. The calculated molar mass gives a reasonable formula for dinitrogen monoxide.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title dinitrogen monoxide gas molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.