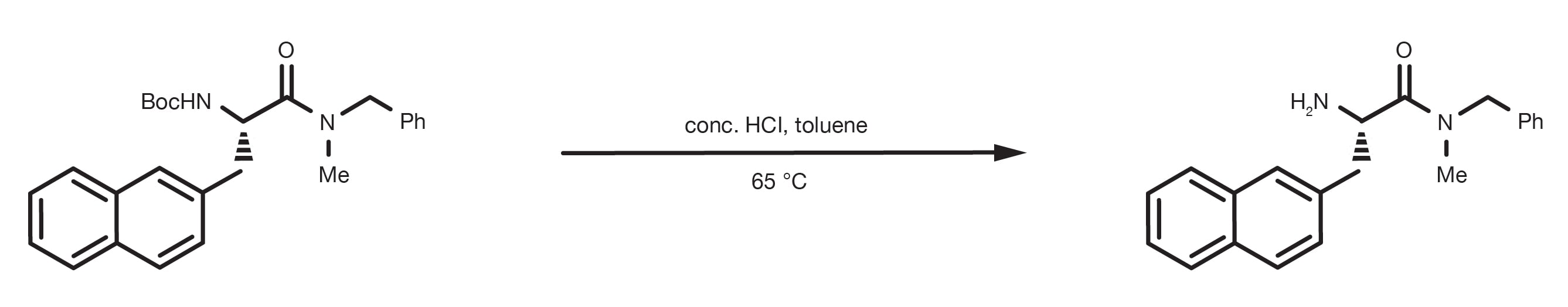
Ethyl carbamate deprotection acid water
Ethyl Carbamate Deprotection Acid Water. Mix the protected carbamate to be deprotected with 3 M hydrochloric acid HCl in ethyl acetate for 30 min at ambient temperature Heat the carbamate in a mixture of aqueous hydrochloric acid and toluene at 65 C. Carbamate ester Carbamic acid Carbamide Carbanion Carbene Carbenoid Carbocation Carbocation fates. The elution started with 60 mobile phase A ultrapure water 01 formic acid and 40 mobile phase B 100 methanol 01 formic acid then held at 40 B for 05 min raised to 95 B in 55 min held at 95 B for 1 min and then lowered to 40 B in 1 min. On silica 구리 ii 테트라플루오로붕산염 수화물copper ii tetrafluoroborate hydrate 구리 - 포스포리즈드copper - phosphorized 구리 - cr hr 구리 리드텍스 층copper - cr hr copper leadtex sheet 구리 1 반응물copper 1 reagent 구리 1xxx 계열copper 1xxx series 구리 2 반응물copper 2 reagent 구리 34 세빈 5 황 80 피넛 분진.

Dissolving desired protected compound in a 5050 mix of dichloromethane and. The reactions are high yielding and the workup is convenient. The reaction mixture was refluxed for about 1 h. Acid Deoxyribose Deprotection Deprotonate Deprotonization DEPT Deshielded Desiccation Desolvation Destructive interference. Exploring the Potential of 2-2-Nitrophenylethyl-Caged N-Hydroxysulfonamides for the Photoactivated Release of Nitroxyl HNO. 1-4-Aminobenzyl-1H-imidazole-2-carboxylic acid 48.
Mix the protected carbamate to be deprotected with 3 M hydrochloric acid HCl in ethyl acetate for 30 min at ambient temperature Heat the carbamate in a mixture of aqueous hydrochloric acid and toluene at 65 C.
1-4-Aminobenzyl-1H-imidazole-2-carboxylic acid 48. The LC-MS data were acquired using ESI mode under the following. The organic solution was combined with a solution of the nitrosation reagent NaNO 2 or t BuONO and formic acid before entering a second microreactor to perform the nitrosation process. Acid-Catalyzed Cascade Reaction of 2-Alkylfurans with αβ-Unsaturated Ketones. We would like to show you a description here but the site wont allow us. Sequential treatment with trimethylsilyl iodide then methanol can also be used for Boc deprotection.
 Source: ec.gc.ca
Source: ec.gc.ca
Ethyl 1-4-nitrobenzyl-1H-imidazole-2-carboxylate A 22a 200 mg 073 mmol was taken in EtOH 8 mL and iron powder 122 mg 218 mmol was added at 5055 C followed by NH 4 Cl solution 19 mg 036 mmol in 4 mL water. The LC-MS data were acquired using ESI mode under the following. The reaction is usually fast and happens at room temperature. These carbamate derivatives do not behave as amines which allows. The column was equilibrated by pumping 40 B for 4 min.
 Source: researchgate.net
Source: researchgate.net
The resulting stream was directed into a Zaiput liquid-liquid separator to remove the water-soluble base TEA from the system and avoid the consumption of the nitrosation agent in the second step. Carbamate ester Carbamic acid Carbamide Carbanion Carbene Carbenoid Carbocation Carbocation fates. On silica 구리 ii 테트라플루오로붕산염 수화물copper ii tetrafluoroborate hydrate 구리 - 포스포리즈드copper - phosphorized 구리 - cr hr 구리 리드텍스 층copper - cr hr copper leadtex sheet 구리 1 반응물copper 1 reagent 구리 1xxx 계열copper 1xxx series 구리 2 반응물copper 2 reagent 구리 34 세빈 5 황 80 피넛 분진. CBZ carbamates azetidine benzyl and methyl esters TBDMS and methyl phenyl ethers are tolerated. Exploring the Potential of 2-2-Nitrophenylethyl-Caged N-Hydroxysulfonamides for the Photoactivated Release of Nitroxyl HNO.
 Source: organic-chemistry.org
Source: organic-chemistry.org
The Journal of Organic Chemistry Articles ASAP Note Publication Date. The Journal of Organic Chemistry Articles ASAP Note Publication Date. We would like to show you a description here but the site wont allow us. Di-tert-butyl dicarbonate is a reagent widely used in organic synthesisSince this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl Boc group it is commonly referred to as Boc anhydrideThis pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. The column was equilibrated by pumping 40 B for 4 min.
 Source: sciencedirect.com
Source: sciencedirect.com
These carbamate derivatives do not behave as amines which allows. A Shortcut to 235-Trisubstituted Furans. Ethyl 1-4-nitrobenzyl-1H-imidazole-2-carboxylate A 22a 200 mg 073 mmol was taken in EtOH 8 mL and iron powder 122 mg 218 mmol was added at 5055 C followed by NH 4 Cl solution 19 mg 036 mmol in 4 mL water. 1-4-Aminobenzyl-1H-imidazole-2-carboxylic acid 48. The resulting stream was directed into a Zaiput liquid-liquid separator to remove the water-soluble base TEA from the system and avoid the consumption of the nitrosation agent in the second step.
 Source: sciencedirect.com
Source: sciencedirect.com
Acid-Catalyzed Cascade Reaction of 2-Alkylfurans with αβ-Unsaturated Ketones. The reaction mixture was refluxed for about 1 h. Acid-Catalyzed Cascade Reaction of 2-Alkylfurans with αβ-Unsaturated Ketones. -Butyl5-4-tert-butoxycarbonylamino-2-tritylthiomethylbutanamido-6-22-dimethoxyethyl2-naphthalene-2-ylethylamino-6-oxohexylcarbamate 42 The title compound was prepared from carboxylic acid 30 325 mg 048 mmol and Fmoc-deprotected amine 35 see general protocol B 066 mmol following. Di-tert-butyl dicarbonate is a reagent widely used in organic synthesisSince this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl Boc group it is commonly referred to as Boc anhydrideThis pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives.
 Source: researchgate.net
Source: researchgate.net
Synthesis of carboxylic acid building blocks. A Shortcut to 235-Trisubstituted Furans. The LC-MS data were acquired using ESI mode under the following. The deprotection of a BOC-protected amine is a simple carbamate hydrolysis in acidic conditions. The column was equilibrated by pumping 40 B for 4 min.
 Source: fishersci.co.uk
Source: fishersci.co.uk
The LC-MS data were acquired using ESI mode under the following. The column was equilibrated by pumping 40 B for 4 min. Synthesis of carboxylic acid building blocks. These carbamate derivatives do not behave as amines which allows. Acid Deoxyribose Deprotection Deprotonate Deprotonization DEPT Deshielded Desiccation Desolvation Destructive interference.
 Source: researchgate.net
Source: researchgate.net
The column was equilibrated by pumping 40 B for 4 min. The column was equilibrated by pumping 40 B for 4 min. These carbamate derivatives do not behave as amines which allows. The resulting stream was directed into a Zaiput liquid-liquid separator to remove the water-soluble base TEA from the system and avoid the consumption of the nitrosation agent in the second step. Di-tert-butyl dicarbonate is a reagent widely used in organic synthesisSince this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl Boc group it is commonly referred to as Boc anhydrideThis pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Ethyl 1-4-nitrobenzyl-1H-imidazole-2-carboxylate A 22a 200 mg 073 mmol was taken in EtOH 8 mL and iron powder 122 mg 218 mmol was added at 5055 C followed by NH 4 Cl solution 19 mg 036 mmol in 4 mL water. The resulting stream was directed into a Zaiput liquid-liquid separator to remove the water-soluble base TEA from the system and avoid the consumption of the nitrosation agent in the second step. Aqueous phosphoric acid is an effective environmentally benign selective and mild reagent for the deprotection of tert-butyl carbamates tert-butyl esters and tert-butyl ethers. We would like to show you a description here but the site wont allow us. The LC-MS data were acquired using ESI mode under the following.

Acid Deoxyribose Deprotection Deprotonate Deprotonization DEPT Deshielded Desiccation Desolvation Destructive interference. The LC-MS data were acquired using ESI mode under the following. -Butyl5-4-tert-butoxycarbonylamino-2-tritylthiomethylbutanamido-6-22-dimethoxyethyl2-naphthalene-2-ylethylamino-6-oxohexylcarbamate 42 The title compound was prepared from carboxylic acid 30 325 mg 048 mmol and Fmoc-deprotected amine 35 see general protocol B 066 mmol following. The elution started with 60 mobile phase A ultrapure water 01 formic acid and 40 mobile phase B 100 methanol 01 formic acid then held at 40 B for 05 min raised to 95 B in 55 min held at 95 B for 1 min and then lowered to 40 B in 1 min. On silica 구리 ii 테트라플루오로붕산염 수화물copper ii tetrafluoroborate hydrate 구리 - 포스포리즈드copper - phosphorized 구리 - cr hr 구리 리드텍스 층copper - cr hr copper leadtex sheet 구리 1 반응물copper 1 reagent 구리 1xxx 계열copper 1xxx series 구리 2 반응물copper 2 reagent 구리 34 세빈 5 황 80 피넛 분진.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title ethyl carbamate deprotection acid water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.