Formula for dinitrogen pentaoxide
Formula For Dinitrogen Pentaoxide. Write the formula for the ionic compound that forms between each pair of elements. Strontium and iodine BeCl2. Calculate the number of moles in 579 g of sodium perchlorate. 83527-55-3 As 4 S 3.
 Dinitrogen Pentoxide Wikipedia From en.wikipedia.org
Dinitrogen Pentoxide Wikipedia From en.wikipedia.org
Write the formula for the ionic compound that forms between each pair of elements. Mg3N2 Magnesium Nitride 2. Handbook of Reactive Chemical Hazards. So the formula is written as SnS2. Name each ionic compound. 1 Structures Expand this section.
Calculate the number of moles in 579 g of sodium perchlorate.
2 Names and Identifiers Expand this section. Sodium and oxygen Na2O 4. To balance the 4 charge on tin we need two sulfide ions. O 0366 g N 2 O 5 _ 064 g NO 5 2 _ 2 10 23 g N 2 O 5 _ 3836 10 23 g NO 5 2. Calculate the number of formula units. So the formula is written as SnS2.

Calculate the mass in grams of 204 10 21 molecules of dinitrogen pentaoxide. 83527-55-3 As 4 S 3. Dinitrogen pentaoxide is a nitrogen oxide. Calculate the number of moles in 579 g of sodium perchlorate. Name each ionic compound.
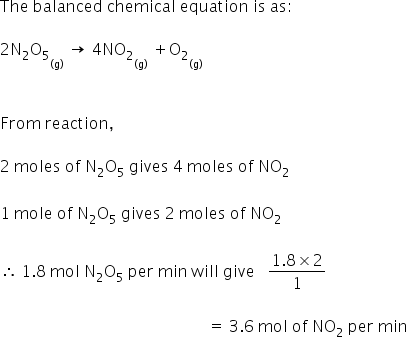 Source: topperlearning.com
Source: topperlearning.com
Name each ionic compound. Strontium and iodine BeCl2. O 0473 mol NaClO 4 _ 054 mol NaClO 3 _ 06 mol NaClO 2 _ 0778 mol NaClO. Handbook of Reactive Chemical Hazards. 2 Names and Identifiers Expand this section.
 Source: youtube.com
Source: youtube.com
Butterworth-Heinemann Ltd 1990 p. Butterworth-Heinemann Ltd 1990 p. 12512-13-9 As 4 S 4. O 0473 mol NaClO 4 _ 054 mol NaClO 3 _ 06 mol NaClO 2 _ 0778 mol NaClO. Mg3N2 Magnesium Nitride 2.
 Source: howtodiscuss.com
Source: howtodiscuss.com
3 Chemical and Physical Properties Expand. The periodic table tells us that sulfide has a 2 charge. Strontium and iodine BeCl2. Handbook of Reactive Chemical Hazards. Sodium and oxygen Na2O 4.
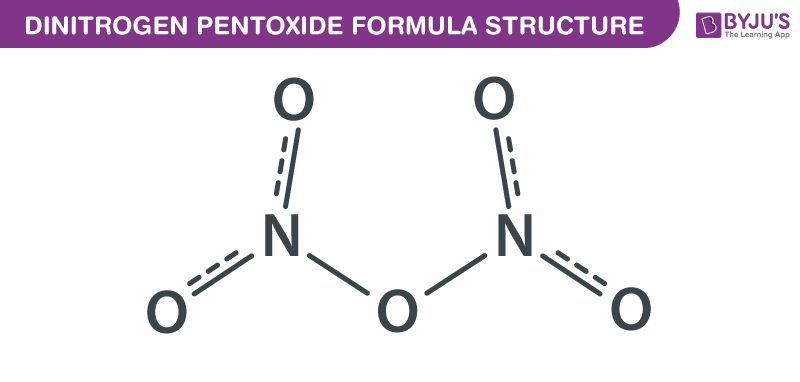 Source: byjus.com
Source: byjus.com
The subscripts in the formula represent the number of positive and negative ions that give an overall charge of zero. Strontium and iodine BeCl2. Aluminum and sulfur Al2S3 2. Dinitrogen pentaoxide is a nitrogen oxide. 1 Structures Expand this section.
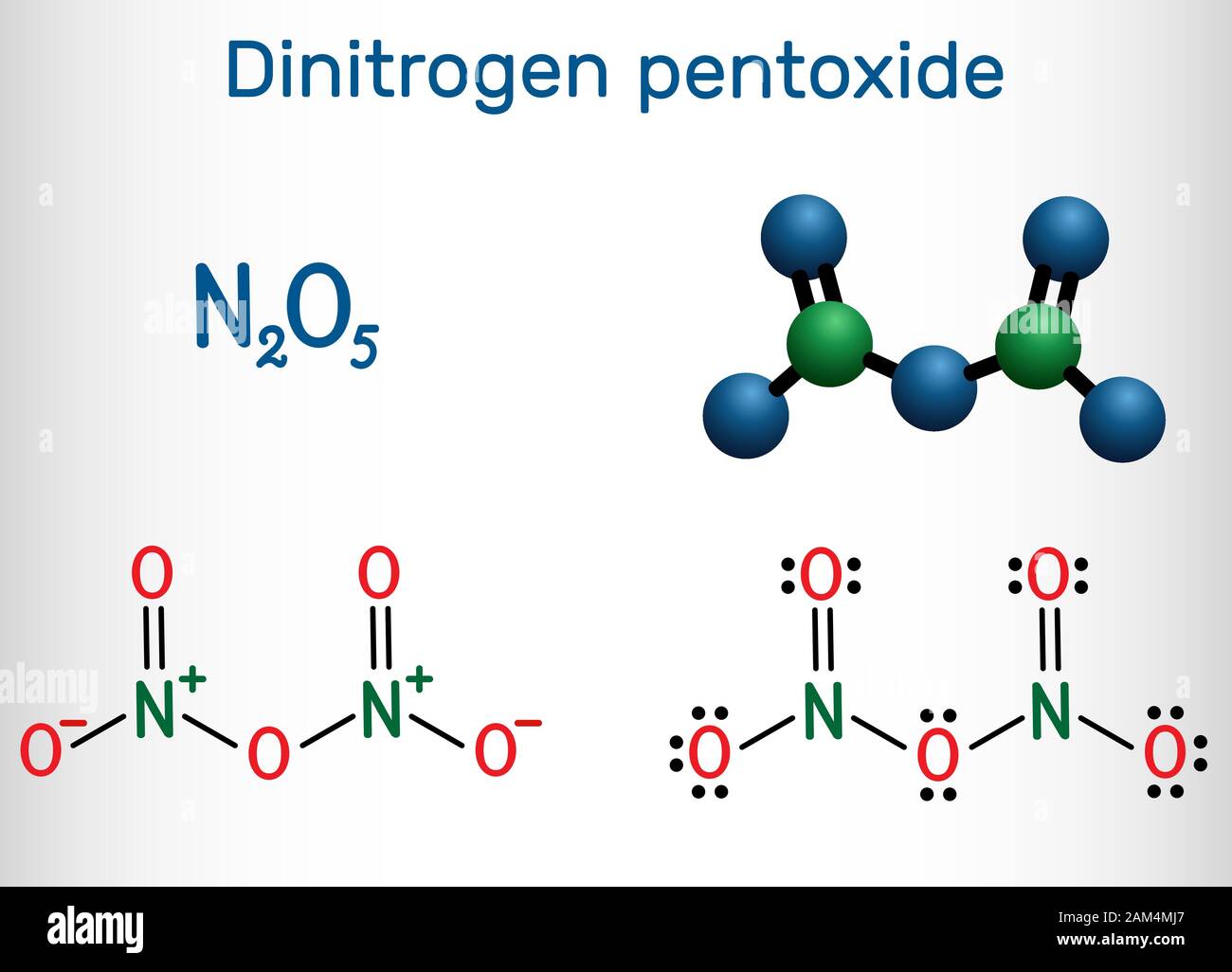 Source: alamy.com
Source: alamy.com
83527-55-3 As 4 S 3. Aluminum and sulfur 2. The periodic table tells us that sulfide has a 2 charge. 83527-55-3 As 4 S 3. 3 Chemical and Physical Properties Expand.
 Source: barewalls.com
Source: barewalls.com
Handbook of Reactive Chemical Hazards. N 2 O 5. Calculate the number of moles in 579 g of sodium perchlorate. Aluminum and sulfur Al2S3 2. Sodium and oxygen Na2O 4.
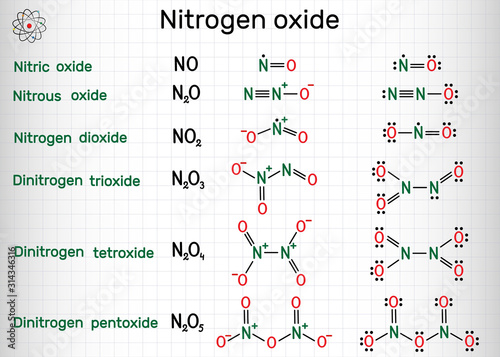 Source: wallsheaven.com
Source: wallsheaven.com
3 Chemical and Physical Properties Expand. So the formula is written as SnS2. Calculate the number of formula units. Sodium and oxygen 4. O 0473 mol NaClO 4 _ 054 mol NaClO 3 _ 06 mol NaClO 2 _ 0778 mol NaClO.
 Source: en.wikipedia.org
Source: en.wikipedia.org
83527-55-3 As 4 S 3. Handbook of Reactive Chemical Hazards. Mg3N2 Magnesium Nitride 2. Sodium and oxygen 4. Strontium and iodine BeCl2.
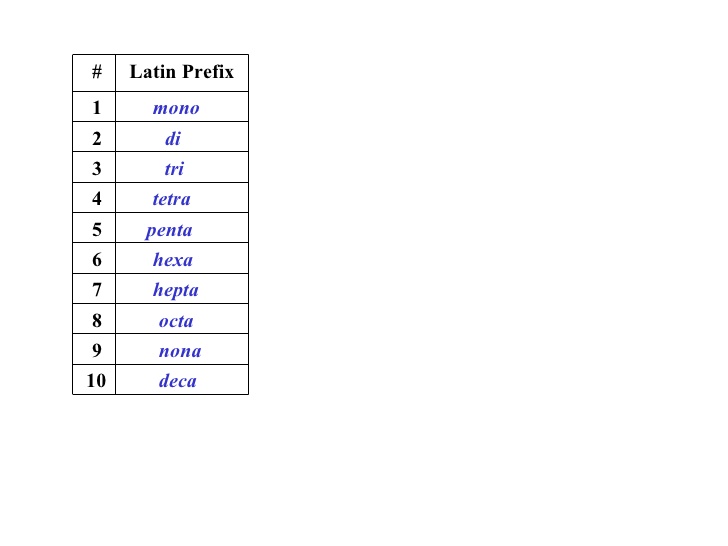 Source: socratic.org
Source: socratic.org
What is the. Calculate the mass in grams of 204 10 21 molecules of dinitrogen pentaoxide. Aluminum and oxygen Al2O 3. 2 Names and Identifiers Expand this section. Aluminum and sulfur 2.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title formula for dinitrogen pentaoxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






