Formula of beryllium oxide
Formula Of Beryllium Oxide. 27 beryllium hydroxide BeOH2 28 zinc carbonate ZnCO3 29 manganese VII arsenide Mn3As7 30 copper II chlorate CuClO32 31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42 35. Use the atomic number to indicate the number of atoms of each type present in the compound. Cations Anions zinc iron II iron III gallium silver lead IV chloride. An ionic compound is composed of a metal and a non-metal.
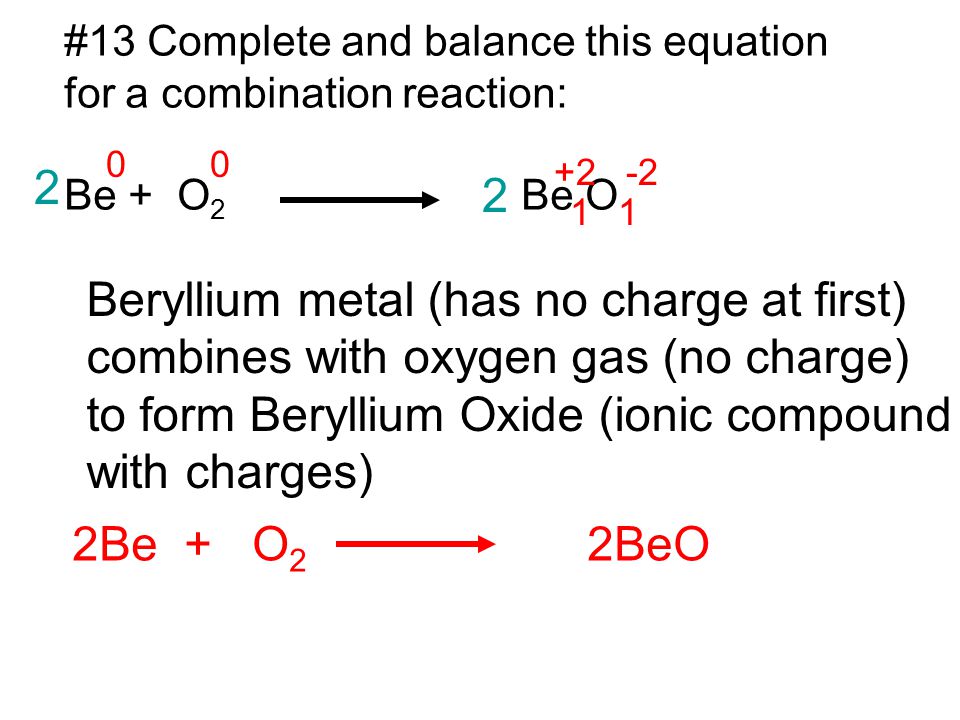 Beryllium Metal Has No Charge At First Ppt Video Online Download From slideplayer.com
Beryllium Metal Has No Charge At First Ppt Video Online Download From slideplayer.com
Metals lose electrons to produce positve ions called cations. Compounds of beryllium are mostly covalent because of high positive charge density on Be 2 ion and polarising power. This colourless solid is a notable electrical insulator with a higher thermal conductivity than any other non-metal except diamond and exceeds that of most metals. Use the atomic number to indicate the number of atoms of each type present in the compound. In nature pure beryl is colorless but acquires its coloration from trace amounts of additional. Beryllium oxide is also being studied for use in increasing the thermal conductivity of uranium dioxide nuclear fuel pellets.
An ionic compound is composed of a metal and a non-metal.
Use the stock form for the transition metals. Sodium chloride NaCl and magnesium oxide MgO. Sodium thiosulfate Na2S2O3_ 3. Beryllium is alloyed with nickel 2 beryllium 98 nickel to make springs spot-welding electrodes and non-sparking tools. Ionic compounds tend to form crystals with high melting temperatures. Except for beryllium the alkaline earth metals react with hydrogen upon heating to form metal hydrides Beryllium does not liberate hydrogen from dilute acid readily while other alkaline earth metals evolved hydrogen.
 Source: youtube.com
Source: youtube.com
Complete these in lab and on your own time for practice. Write the metal first and the non-metal second. An ionic compound is composed of a metal and a non-metal. Use the atomic number to indicate the number of atoms of each type present in the compound. Use the stock form for the transition metals.
Source: chemspider.com
The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. Red Beryl Be 3 Al 2 Si 6 O 18 is a mineral composed of beryllium aluminum and silicate. The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times. Chemical Formula Nomenclature Practice. Sodium chloride NaCl and magnesium oxide MgO.
Source: yeahchemistry.com
Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N. Metals lose electrons to produce positve ions called cations. Compounds of beryllium are mostly covalent because of high positive charge density on Be 2 ion and polarising power. Except for beryllium the alkaline earth metals react with hydrogen upon heating to form metal hydrides Beryllium does not liberate hydrogen from dilute acid readily while other alkaline earth metals evolved hydrogen. Complete these in lab and on your own time for practice.
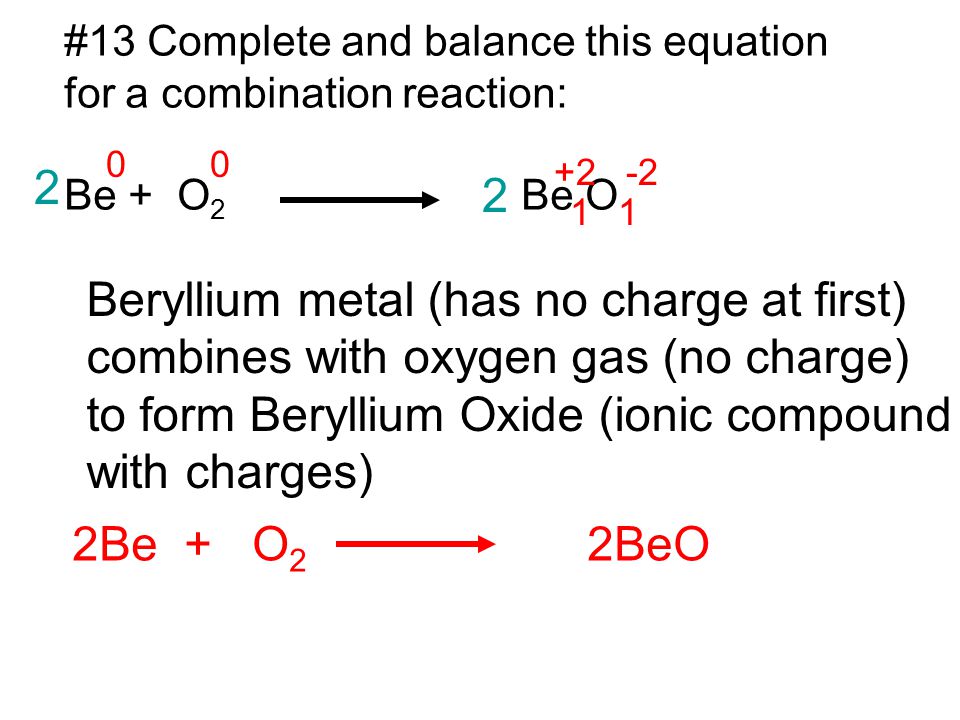 Source: slideplayer.com
Source: slideplayer.com
Beryllium compounds were used in fluorescent lighting tubes but this use was discontinued because of the disease berylliosis which developed in the. The transfer of electrons between metals and non-metals produces charged particles called ions. Ionic compounds tend to form crystals with high melting temperatures. The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times. From 1998 to 2000 the McLaren Formula One team used Mercedes-Benz engines with beryllium-aluminium-alloy pistons.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Use the stock form for the transition metals. Red Beryl Be 3 Al 2 Si 6 O 18 is a mineral composed of beryllium aluminum and silicate. The transfer of electrons between metals and non-metals produces charged particles called ions. Na Mg 2 Non. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
 Source: youtube.com
Source: youtube.com
Other beryllium alloys are used in the windshield brake disks and other structural components of the space shuttle. An ionic compound is composed of a metal and a non-metal. This colourless solid is a notable electrical insulator with a higher thermal conductivity than any other non-metal except diamond and exceeds that of most metals. 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3 As 30 iron III oxide Fe 2 O 3 31 gallium nitride GaN 32 iron II bromide FeBr 2 33 vanadium V phosphate V 3 PO 4 5 34 calcium oxide CaO 35 magnesium acetate MgCH 3 COO 2 36 aluminum sulfate Al. 27 beryllium hydroxide BeOH2 28 zinc carbonate ZnCO3 29 manganese VII arsenide Mn3As7 30 copper II chlorate CuClO32 31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42 35.
 Source: youtube.com
Source: youtube.com
Na Mg 2 Non. Compounds of beryllium are mostly covalent because of high positive charge density on Be 2 ion and polarising power. Beryllium is alloyed with nickel 2 beryllium 98 nickel to make springs spot-welding electrodes and non-sparking tools. An ionic compound is composed of a metal and a non-metal. Chemical Formula Nomenclature Practice.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Beryllium oxide BeO a compound of beryllium is used in the nuclear industry and in ceramics. Other beryllium alloys are used in the windshield brake disks and other structural components of the space shuttle. Beryllium compounds were used in fluorescent lighting tubes but this use was discontinued because of the disease berylliosis which developed in the. Beryllium oxide BeO is of amphoteric nature. This colourless solid is a notable electrical insulator with a higher thermal conductivity than any other non-metal except diamond and exceeds that of most metals.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Except for beryllium the alkaline earth metals react with hydrogen upon heating to form metal hydrides Beryllium does not liberate hydrogen from dilute acid readily while other alkaline earth metals evolved hydrogen. Sulfur dioxide SO2_ 2. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. Sodium chloride NaCl and magnesium oxide MgO. Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N.
 Source: youtube.com
Source: youtube.com
Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N. Compounds of beryllium are mostly covalent because of high positive charge density on Be 2 ion and polarising power. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. An ionic compound is composed of a metal and a non-metal. The names are found by finding the intersection between the cations and anions.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title formula of beryllium oxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







