Formula of mercury oxide
Formula Of Mercury Oxide. Metals lose electrons to produce positve ions called cations. Calcium oxide CaO commonly known as quicklime or burnt lime is a widely used chemical compoundIt is a white caustic alkaline crystalline solid at room temperature. Cs2S cesium sulfide 42. Hg23N2 mercury I nitride 45.

SO 4 2. The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing. The broadly used term lime connotes calcium-containing inorganic materials in which carbonates oxides and hydroxides of calcium silicon magnesium aluminium and iron predominate. Parotid-Lyph 60 tablets per bottle. Metals lose electrons to produce positve ions called cations. PbCl4 lead IV chloride 41.
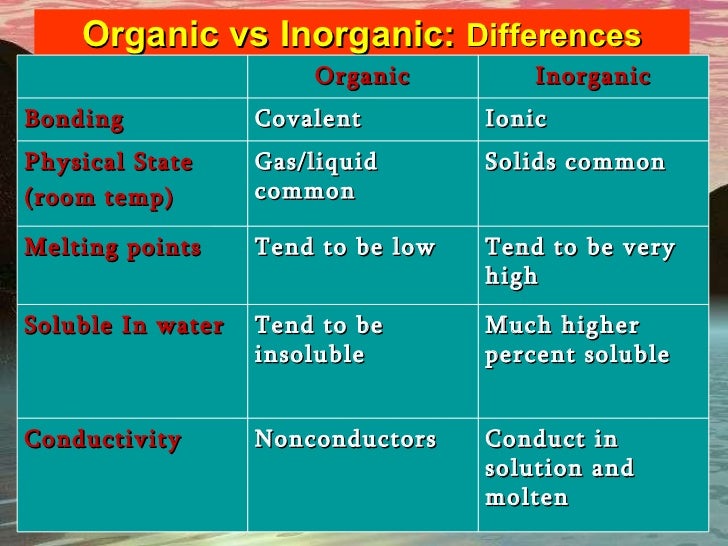
For example in NaCl the sodium atom acts as the cation while the chlorine atom acts as the anion.
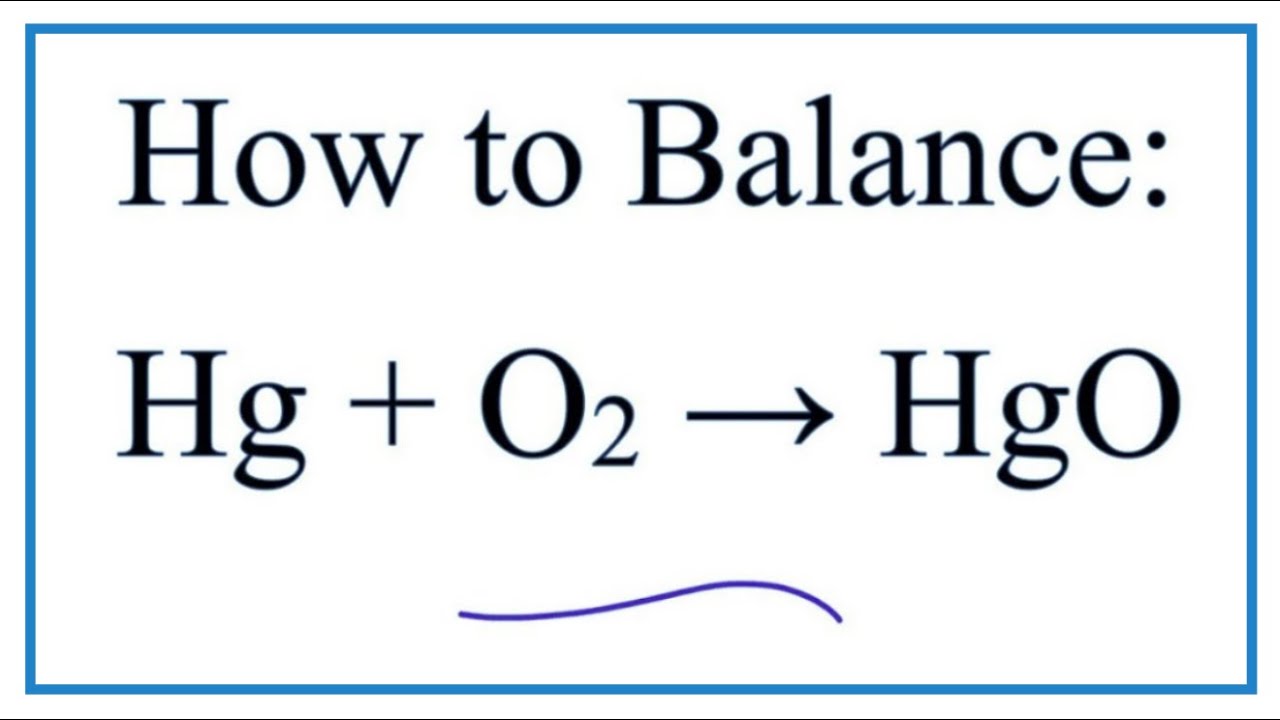
2 Determine the moles then mass of O 2 produced from the decomposition. Hg2O mercury I oxide 35. LiBr lithium bromide 39. FeS iron II sulfide 44. 1761 1545 216 g. 2 Determine the moles then mass of O 2 produced from the decomposition.
 Source: youtube.com
Source: youtube.com
Pantothenic Acid 90 tablets per bottle. From the results of the experiment determine the molecular formula of this oxide of mercury Solution. Hg23N2 mercury I nitride 45. Pantothenic Acid 90 tablets per bottle. Our purpose is to solve the toughest problems in life science by collaborating with the global scientific community and through that we aim to accelerate access to better health for people everywhere.

History- The type of naming you will learn about is called the Stock system or Stocks system. From the results of the experiment determine the molecular formula of this oxide of mercury Solution. FeI2 iron II iodide 38. SO 4 2. Pantothenic Acid 90 tablets per bottle.
 Source: youtube.com
Source: youtube.com
Such as on combining H and PO43- the charges are crossed over to form H3PO4. 1761 1545 216 g. Calculate the empirical formula. Hg23N2 mercury I nitride 45. Penta-Cal-Plus 500 500 tablets per bottle.

2 Determine the moles then mass of O 2 produced from the decomposition. Mercuric oxide solid appears as red or orange-red odorless dense crystalline powder or scales yellow when finely powdered. The broadly used term lime connotes calcium-containing inorganic materials in which carbonates oxides and hydroxides of calcium silicon magnesium aluminium and iron predominate. Metals lose electrons to produce positve ions called cations. Hg23N2 mercury I nitride 45.

2 Determine the moles then mass of O 2 produced from the decomposition. 1 Mass of oxide. The compound benzamide has the following percent composition. Mass of test tube oxide of mercury 1761 g Volume of oxygen collected at RTP 120. Pantothenic Acid 90 tablets per bottle.
 Source: youtube.com
Source: youtube.com
SrCl2 strontium chloride 36. Sodium chloride NaCl and magnesium oxide MgO. Writing Chemical Formulas. Calcium oxide CaO commonly known as quicklime or burnt lime is a widely used chemical compoundIt is a white caustic alkaline crystalline solid at room temperature. SrO strontium oxide 40.
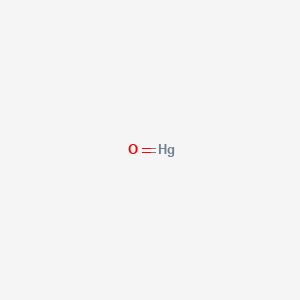
RTP has the following values. Writing Chemical Formulas. Penta-Cal-Plus 250 250 tablets per bottle. It was designed by Alfred Stock 1876-1946 a German chemist and first published in 1919. The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing.
 Source: youtube.com
Source: youtube.com
Penta-Cal-Plus 250 250 tablets per bottle. MercuryII oxide also called mercuric oxide or simply mercury oxide has a formula of Hg O. When writing the formula of a compound the cation is listed before the anion. History- The type of naming you will learn about is called the Stock system or Stocks system. Pedia-C 60 tablets per bottle.
 Source: shutterstock.com
Source: shutterstock.com
Calcium oxide CaO commonly known as quicklime or burnt lime is a widely used chemical compoundIt is a white caustic alkaline crystalline solid at room temperature. Al2O3 aluminum oxide 37. From the results of the experiment determine the molecular formula of this oxide of mercury Solution. O 2 -Sulfate anion. 1 Mass of oxide.

FeS iron II sulfide 44. An ionic compound is composed of a metal and a non-metal. FeS iron II sulfide 44. Pantothenic Acid 90 tablets per bottle. SO 4 2.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title formula of mercury oxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.