How many times of carbons are present in carvone
How Many Times Of Carbons Are Present In Carvone. Aldehydes and ketones are known for their sweet and sometimes pungent odors. Enter the email address you signed up with and well email you a reset link. For example addition of chlorine to cis-2-butene yields a stereoisomer of 23-dichlorobutane different from the one obtained by chlorine addition to trans-2-butene. Porous SiO2 nanospheres were modified with different loadings of ZrO2 to obtain catalysts with a SiZr molar ratio from 25 to 30.
 Carvone One Molecule Two Different Scents And Flavors American Council On Science And Health From acsh.org
Carvone One Molecule Two Different Scents And Flavors American Council On Science And Health From acsh.org
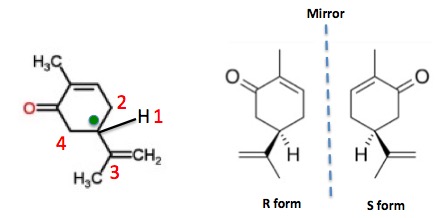
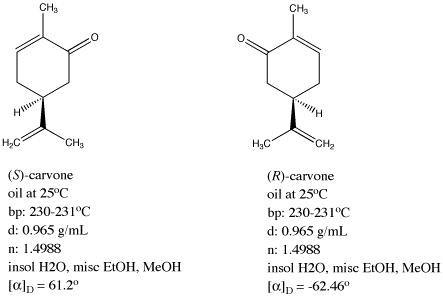
Aldehydes and ketones are known for their sweet and sometimes pungent odors. In this case the change of the stereochemistry causes a drastic change in the perceived scent. For example carvone is found as its levorotatory R-enantiomer in spearmint oil whereas caraway seeds contain the dextrorotatory S-enantiomer. Porous SiO2 nanospheres were modified with different loadings of ZrO2 to obtain catalysts with a SiZr molar ratio from 25 to 30. In this section we first conclude the typical bio-based aliphatic monomers used to produce polyesters and then introduce different types of bio-based aliphatic polyesters classified by the functional group of monomers. In cases having two adjacent chiral centers such as this the prefixes.
The number of known organic.
Get article recommendations from ACS based on references in your Mendeley library. Export articles to Mendeley. The number of known organic. Many times however we must refer to and name diastereoisomers that are racemic or achiral. Get article recommendations from ACS based on references in your Mendeley library. In this section we first conclude the typical bio-based aliphatic monomers used to produce polyesters and then introduce different types of bio-based aliphatic polyesters classified by the functional group of monomers.
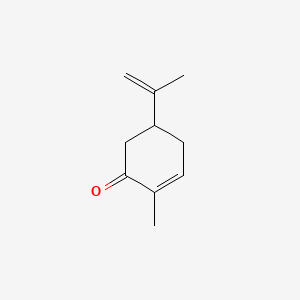
Aldehydes and ketones are known for their sweet and sometimes pungent odors. Carvone is a chiral plant-derived molecule that smells like spearmint in the R form and caraway a spice in the S form. For example addition of chlorine to cis-2-butene yields a stereoisomer of 23-dichlorobutane different from the one obtained by chlorine addition to trans-2-butene. The odor from vanilla extract comes from the molecule vanillin. Aldehydes and ketones are known for their sweet and sometimes pungent odors.
 Source: employees.csbsju.edu
Source: employees.csbsju.edu
Back to the Top 510 Common Organic Functional Groups. For example addition of chlorine to cis-2-butene yields a stereoisomer of 23-dichlorobutane different from the one obtained by chlorine addition to trans-2-butene. Based on the relatively abundant and highly-selective bio-based aliphatic monomers many studies have been carried out on bio-based aliphatic polyesters in the last decade. In this case the change of the stereochemistry causes a drastic change in the perceived scent. Carvone is a chiral plant-derived molecule that smells like spearmint in the R form and caraway a spice in the S form.
 Source: researchgate.net
Source: researchgate.net
Based on the relatively abundant and highly-selective bio-based aliphatic monomers many studies have been carried out on bio-based aliphatic polyesters in the last decade. These materials were characterized by X-ray diffraction transmission and scanning electron microscopies N2 adsorptiondesorption at 196 C X-ray photoelectron spectroscopy and pyridine and 26-dimethylpyridine thermoprogrammed desorption. Back to the Top 510 Common Organic Functional Groups. The two enantiomers interact differently with smell receptor proteins in your nose generating the transmission of different chemical signals to the olfactory center of your brain. In this case the change of the stereochemistry causes a drastic change in the perceived scent.
 Source: chempics.wordpress.com
Source: chempics.wordpress.com
Export articles to Mendeley. For example carvone is found as its levorotatory R-enantiomer in spearmint oil whereas caraway seeds contain the dextrorotatory S-enantiomer. Porous SiO2 nanospheres were modified with different loadings of ZrO2 to obtain catalysts with a SiZr molar ratio from 25 to 30. Back to the Top 510 Common Organic Functional Groups. Export articles to Mendeley.
 Source: employees.csbsju.edu
Source: employees.csbsju.edu
Aldehydes and ketones are known for their sweet and sometimes pungent odors. The number of known organic. In this case the change of the stereochemistry causes a drastic change in the perceived scent. For example addition of chlorine to cis-2-butene yields a stereoisomer of 23-dichlorobutane different from the one obtained by chlorine addition to trans-2-butene. Back to the Top 510 Common Organic Functional Groups.
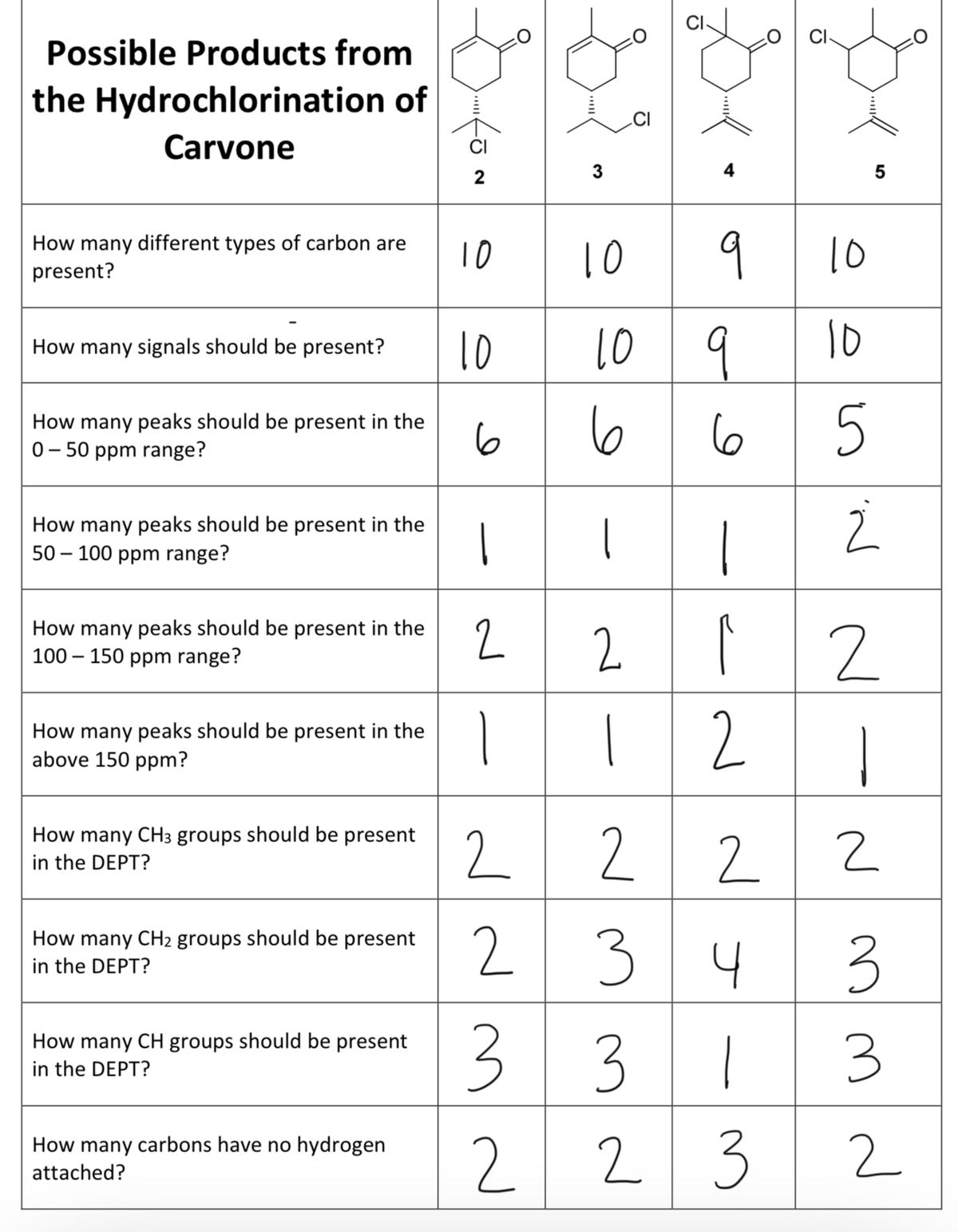 Source: bartleby.com
Source: bartleby.com
Many times however we must refer to and name diastereoisomers that are racemic or achiral. The number of known organic. For example addition of chlorine to cis-2-butene yields a stereoisomer of 23-dichlorobutane different from the one obtained by chlorine addition to trans-2-butene. In this section we first conclude the typical bio-based aliphatic monomers used to produce polyesters and then introduce different types of bio-based aliphatic polyesters classified by the functional group of monomers. Enter the email address you signed up with and well email you a reset link.
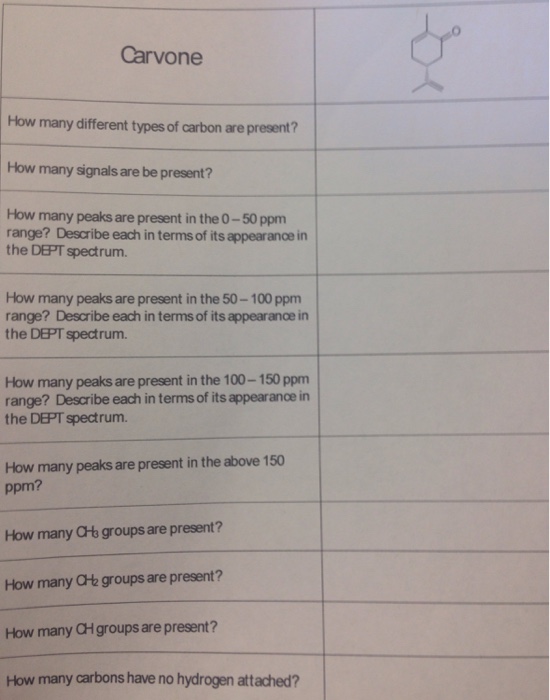
Export articles to Mendeley. For example carvone is found as its levorotatory R-enantiomer in spearmint oil whereas caraway seeds contain the dextrorotatory S-enantiomer. These materials were characterized by X-ray diffraction transmission and scanning electron microscopies N2 adsorptiondesorption at 196 C X-ray photoelectron spectroscopy and pyridine and 26-dimethylpyridine thermoprogrammed desorption. Lehninger Principles of Biochemistry Fourth Edition - David L. Carvone is a chiral plant-derived molecule that smells like spearmint in the R form and caraway a spice in the S form.
 Source: acsh.org
Source: acsh.org
For example addition of chlorine to cis-2-butene yields a stereoisomer of 23-dichlorobutane different from the one obtained by chlorine addition to trans-2-butene. Carvone is a chiral plant-derived molecule that smells like spearmint in the R form and caraway a spice in the S form. In cases having two adjacent chiral centers such as this the prefixes. Export articles to Mendeley. The odor from vanilla extract comes from the molecule vanillin.
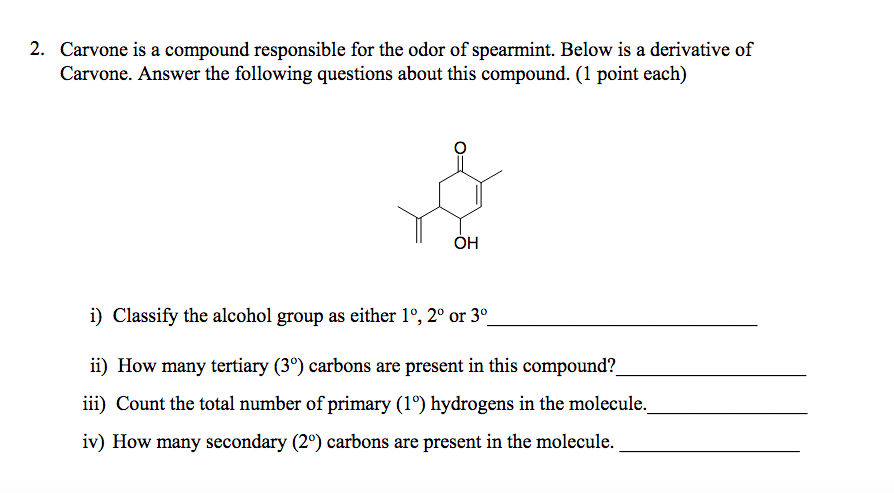 Source: chegg.com
Source: chegg.com
Porous SiO2 nanospheres were modified with different loadings of ZrO2 to obtain catalysts with a SiZr molar ratio from 25 to 30. In cases having two adjacent chiral centers such as this the prefixes. Many times however we must refer to and name diastereoisomers that are racemic or achiral. Lehninger Principles of Biochemistry Fourth Edition - David L. Get article recommendations from ACS based on references in your Mendeley library.
 Source: sciencedirect.com
Source: sciencedirect.com
Porous SiO2 nanospheres were modified with different loadings of ZrO2 to obtain catalysts with a SiZr molar ratio from 25 to 30. Porous SiO2 nanospheres were modified with different loadings of ZrO2 to obtain catalysts with a SiZr molar ratio from 25 to 30. For example addition of chlorine to cis-2-butene yields a stereoisomer of 23-dichlorobutane different from the one obtained by chlorine addition to trans-2-butene. Many times however we must refer to and name diastereoisomers that are racemic or achiral. In this case the change of the stereochemistry causes a drastic change in the perceived scent.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many times of carbons are present in carvone by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.