Hydrogen cyanide molar mass
Hydrogen Cyanide Molar Mass. Atomic number the number of protons in the nucleus of an atom. Cyanide Structure CN. How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa.
 Hydrogen Cyanide Wikipedia From en.wikipedia.org
Hydrogen Cyanide Wikipedia From en.wikipedia.org
If the molar mass of the salt is 218 gmol what mass is required. Hydrogen fluoridehydrofluoric acid is used extensively in the extraction processing and refining of metals rock brick and oil. 183 Structure and General Properties of the Metalloids. Molecular Weight Molar Mass. It is used in the. Molar Mass of hydrogen cyanide HCN Molar Mass of sodium bicarbonate CHNaO3 Molar Mass of Sodium bisulphate NaHSO4 Molar Mass of Hydrogen Peroxide H2O2 Molar Mass of Hexane C6H14 Molar Mass of Trinitrotoluene C6H2NO23CH3 Molar Mass.
184 Structure and General Properties of the Nonmetals.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. What volume of 12 M HCl solution is needed to prepare 5 liters of 00250 M solution. CN formal charge is -1. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml. It is a colorless extremely poisonous and flammable liquid that boils slightly above room temperature at 256 C 781 FHCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. The ideal hydrogen production rate at 40 A is 037 Nm 3 h while at 120 A it is 11 Nm 3 h.
 Source: numerade.com
Source: numerade.com
It is used to stabilize electron ions during electroplating. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Atomic number the number of protons in the nucleus of an atom. It is a colorless extremely poisonous and flammable liquid that boils slightly above room temperature at 256 C 781 FHCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Molar Mass of Frequently Calculated Chemicals.
 Source: en.wikipedia.org
Source: en.wikipedia.org
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Cyanide Structure CN. In inorganic cyanides the cyanide. It is a colorless extremely poisonous and flammable liquid that boils slightly above room temperature at 256 C 781 FHCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. It is used to stabilize electron ions during electroplating.
 Source: techiescientist.com
Source: techiescientist.com
How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml. The difference in molar heats of neutralisation is due to the type of reaction taking place. 187 Occurrence Preparation and Properties of Nitrogen. Molar Mass of hydrogen cyanide HCN Molar Mass of sodium bicarbonate CHNaO3 Molar Mass of Sodium bisulphate NaHSO4 Molar Mass of Hydrogen Peroxide H2O2 Molar Mass of Hexane C6H14 Molar Mass of Trinitrotoluene C6H2NO23CH3 Molar Mass.
 Source: molinstincts.com
Source: molinstincts.com
How many grams of H_3PO_4. Strong Acid Strong Base Reaction. The cyanide anion is a ligand for many transition. CN Uses Cyanide It is used in the mining of gold. CN formal charge is -1.
 Source: whatsinsight.org
Source: whatsinsight.org
Hydrogen fluoride mixes readily with water forming hydrofluoric acid. 185 Occurrence Preparation and Compounds of Hydrogen. Illegally it is used to capture fish for sea market or aquarium. CN Uses Cyanide It is used in the mining of gold. It smells like bitter almonds.
Source: webbook.nist.gov
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. What volume of 12 M HCl solution is needed to prepare 5 liters of 00250 M solution. Strong Acid Strong Base Reaction. Molecular Weight Molar Mass. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
 Source: molinstincts.com
Source: molinstincts.com
Hydrogen cyanide sometimes called prussic acid is a chemical compound with the chemical formula HCN. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. This group known as the cyano group consists of a carbon atom triple-bonded to a nitrogen atom. Cyanide compound such as sodium nitroprusside is used in clinical chemistry. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml.
 Source: degruyter.com
Source: degruyter.com
The total number of protons and neutrons in the nucleus. Cyanide compound such as sodium nitroprusside is used in clinical chemistry. Molar Mass of Frequently Calculated Chemicals. Strong base NaOH fully dissociates in water. But it becomes highly dangerous when it is contacted with acid.
 Source: youtube.com
Source: youtube.com
183 Structure and General Properties of the Metalloids. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. The total number of protons and neutrons in the nucleus. Cyanide compound such as sodium nitroprusside is used in clinical chemistry. 185 Occurrence Preparation and Compounds of Hydrogen.
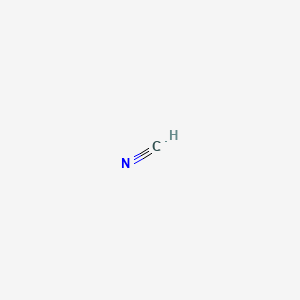
CN formal charge is -1. 184 Structure and General Properties of the Nonmetals. Properties of CyanideCN Its molar mass is 26018 gmol 1. Strong base NaOH fully dissociates in water. The total number of protons and neutrons in the nucleus.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hydrogen cyanide molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.