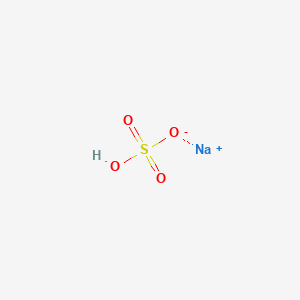
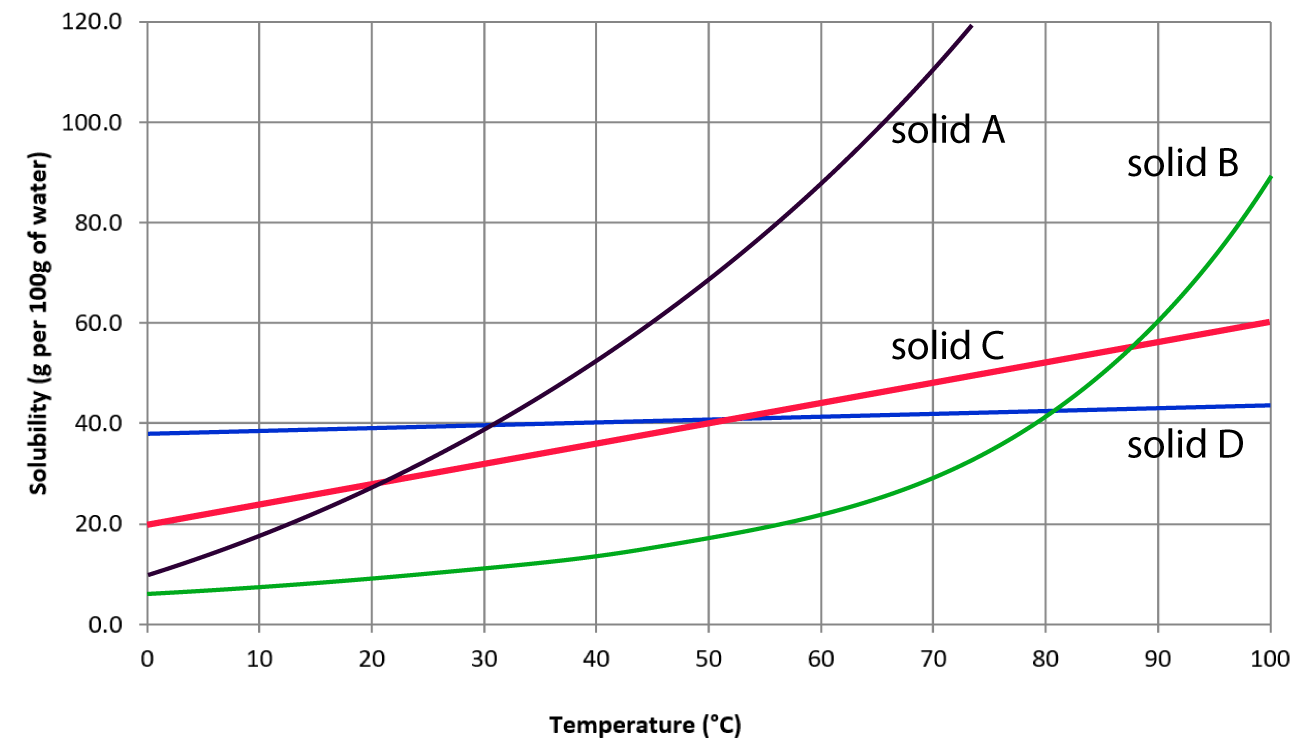
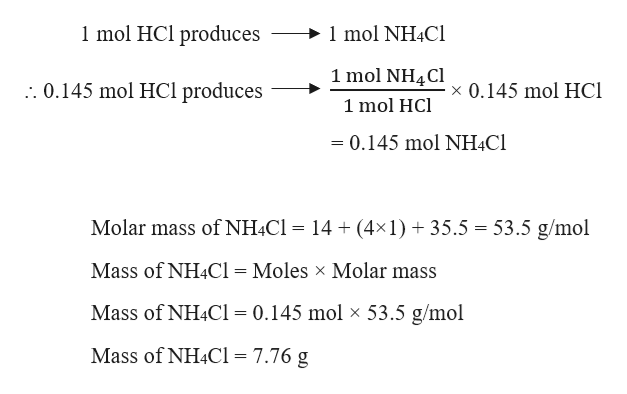
Hydrogen sulfide use
Hydrogen Sulfide Use. National Academy Press Committee on Toxicology Board on Toxicology and. Iron agars to detect H2S This medium is suitable for. Hydrogen sulfide is considered neither a primary nor a secondary contaminant in the Environmental Protection Agencys current drinking water standards but if the concentration of hydrogen sulfide in water is more than 05 parts per million ppm it will. Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas.
 Hydrogen Sulfide Galnet Wiki Fandom From galnet.fandom.com
Hydrogen Sulfide Galnet Wiki Fandom From galnet.fandom.com
Have an unpleasant odor. Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas. Liberates toxic hydrogen sulfide on contact with acids. On contact with acids it liberates highly toxic and flammable hydrogen sulfide gas. Carbonyl sulfide a new hydrophobic and volatile metabolite of disulfiram was found in blood samples from alcoholics treated with single or repeated doses of disulfiramThe dosage schedule included 400 mg of disulfiram every second or third day. Tarnish or discolor silverware.
Liberates toxic hydrogen sulfide on contact with acids.
Pollution prevention and. Pollution prevention and. Beef extract 30 g Peptone 300 g Ferrous ammonium sulphat 02 g Sodium thiosulphate 0025 g Agar 30g Final pH at 25C 7302 Distilled water 1000ml. Lead acetate is toxic to bacteria and may inhibit the growth of some bacteria. Patients with elevated hydrogen sulfide levels may have appeared normal on previous breath tests. Some lots of charcoal have excessively high sulfur backgroun ds andor poor desorption efficiencies.
 Source: sciencedirect.com
Source: sciencedirect.com
Manufacturing Chemists Association pp. The intensity of the blue color is proportional to the sulfide concentration. National Academy Press Committee on Toxicology Board on Toxicology and. Properties and essential information for safe handling and use of hydrogen sulfide. High relative humidity 80 increases the capacity of the sampler four-fold relative to dry air.
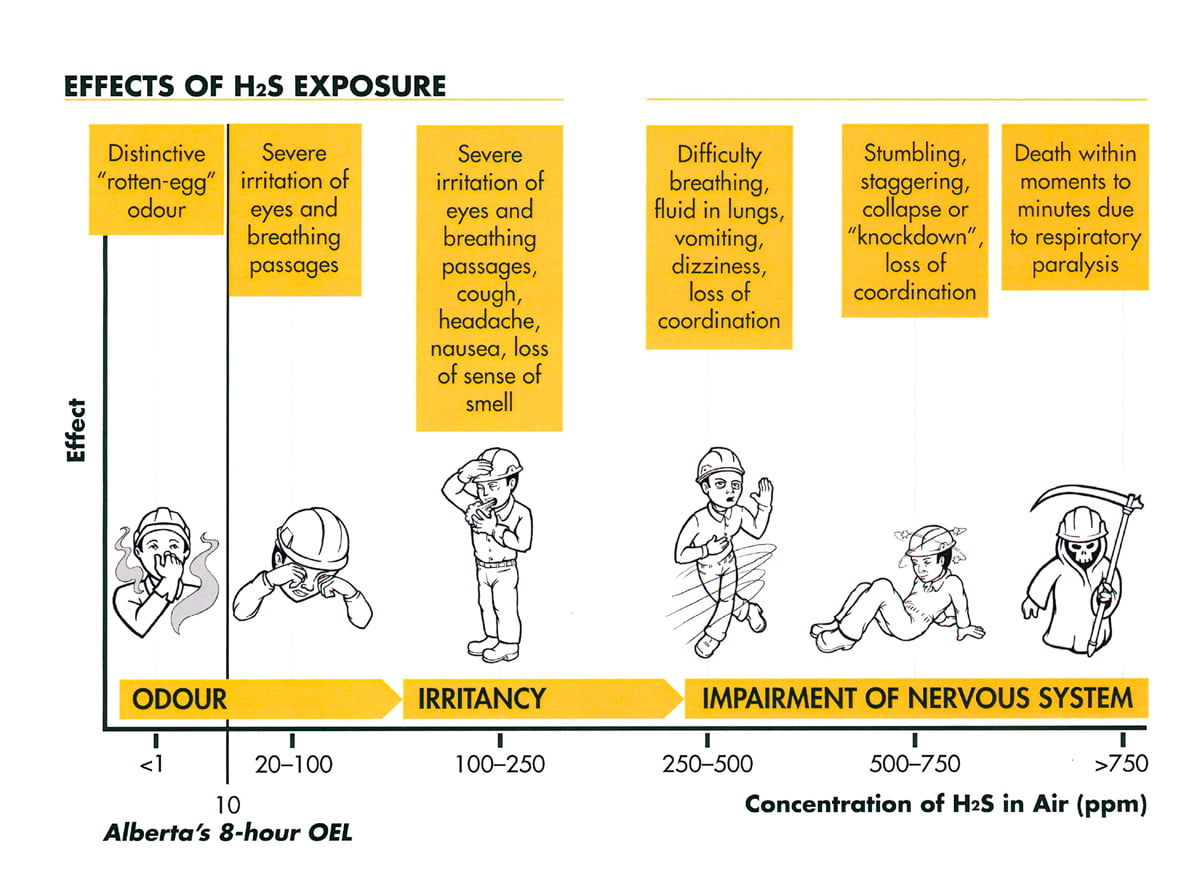 Source: hnhu.org
Source: hnhu.org
On contact with acids it liberates highly toxic and flammable hydrogen sulfide gas. High relative humidity 80 increases the capacity of the sampler four-fold relative to dry air. The efforts have improved the air quality by reducing the hydrogen sulfide emanating from the channel which is the source of the rotten egg odor. Concentrations of hydrogen sulfide and other substances in the air including water vapor. In a recent study subjects with elevated levels of hydrogen sulfide had the greatest diarrhea among subjects referred for testing.
 Source: sci-news.com
Source: sci-news.com
High relative humidity 80 increases the capacity of the sampler four-fold relative to dry air. The efforts have improved the air quality by reducing the hydrogen sulfide emanating from the channel which is the source of the rotten egg odor. H2S production may be inhibited on TSI for organisms that utilize sucrose and suppress the enzyme mechanism that results in the production of H2S. Some lots of charcoal have excessively high sulfur backgroun ds andor poor desorption efficiencies. 54 Likes 13 Comments - UCLA VA Physiatry Residency uclava_pmrresidency on Instagram.
 Source: sciencedirect.com
Source: sciencedirect.com
After an interval of 3 days with no treatment all were given a dose of 400 mg and blood samples taken before ingestion and 4 hr after ingestion. Hydrogen sulfide-positive organisms. High sulfide levels in oil field waters may be determined after proper dilution. The intensity of the blue color is proportional to the sulfide concentration. Liberates toxic hydrogen sulfide on contact with acids.
 Source: acs.org
Source: acs.org
Emergency and continuous exposure guidance levels for selected airborne contaminants. Fast Company inspires a new breed of innovative and creative thought leaders who are actively inventing the future of business. SO 2 is a positive. Hydrogen chloride is a diatomic molecule consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bondThe chlorine atom is much more electronegative than the hydrogen atom which makes this bond polar. H2S production may be inhibited on TSI for organisms that utilize sucrose and suppress the enzyme mechanism that results in the production of H2S.
 Source: en.wikipedia.org
Source: en.wikipedia.org
After an interval of 3 days with no treatment all were given a dose of 400 mg and blood samples taken before ingestion and 4 hr after ingestion. Trio-smart is the only clinical breath test to measure hydrogen sulfide in addition to hydrogen and methane. Properties and essential information for safe handling and use of hydrogen sulfide. 1185 Hazardous Reactivities and Incompatibilities. Hydrogen sulfide is considered neither a primary nor a secondary contaminant in the Environmental Protection Agencys current drinking water standards but if the concentration of hydrogen sulfide in water is more than 05 parts per million ppm it will.
 Source: chemistryworld.com
Source: chemistryworld.com
Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas. Manufacturing Chemists Association pp. Carbonyl sulfide a new hydrophobic and volatile metabolite of disulfiram was found in blood samples from alcoholics treated with single or repeated doses of disulfiramThe dosage schedule included 400 mg of disulfiram every second or third day. In a recent study subjects with elevated levels of hydrogen sulfide had the greatest diarrhea among subjects referred for testing. Therefore screening of each lot should be done before field use.
 Source: galnet.fandom.com
Source: galnet.fandom.com
When heated to decomposition it emits toxic fumes of sodium oxide and oxides of sulfur Bretherick 5th ed 1995 p. 1185 Hazardous Reactivities and Incompatibilities. Therefore screening of each lot should be done before field use. High sulfide levels in oil field waters may be determined after proper dilution. When heated to decomposition it emits toxic fumes of sodium oxide and oxides of sulfur Bretherick 5th ed 1995 p.
 Source: energyeducation.ca
Source: energyeducation.ca
Emergency and continuous exposure guidance levels for selected airborne contaminants. Pollution prevention and. Some lots of charcoal have excessively high sulfur backgroun ds andor poor desorption efficiencies. 1185 Hazardous Reactivities and Incompatibilities. When heated to decomposition it emits toxic fumes of sodium oxide and oxides of sulfur Bretherick 5th ed 1995 p.
 Source: en.wikipedia.org
Source: en.wikipedia.org
54 Likes 13 Comments - UCLA VA Physiatry Residency uclava_pmrresidency on Instagram. Salmonella species Proteus mirabilis. When heated to decomposition it emits toxic fumes of sodium oxide and oxides of sulfur Bretherick 5th ed 1995 p. 1185 Hazardous Reactivities and Incompatibilities. In a recent study subjects with elevated levels of hydrogen sulfide had the greatest diarrhea among subjects referred for testing.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hydrogen sulfide use by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.