Is iodine a base or acid
Is Iodine A Base Or Acid. Doors of Durin on the Wall of Moria Future Web Site Hosting. The more stable 2º-carbocation is formed preferentially and the conjugate base of the Brønsted acid chloride anion in the example shown below then rapidly bonds to this electrophilic intermediate to form the final product. The following energy diagram. The result is that the color produced by a starch-iodine complex is more intense than that obtained with a glycogen.
 What Does Addition Of Iodine And Base Do This Case Chemistry Stack Exchange From chemistry.stackexchange.com
What Does Addition Of Iodine And Base Do This Case Chemistry Stack Exchange From chemistry.stackexchange.com
More specifically deprotonating benzoic acid forms a water-soluble carboxylate anion leaving the other organic by-products in the organic layer. The following energy diagram. However when all ascorbic acid was utilized expected blue-black. Periodic acid ˌ p ɜːr aɪ ˈ ɒ d ɪ k per-eye-OD-ik is the highest oxoacid of iodine in which the iodine exists in oxidation state 7. Orthoperiodic acid with the chemical formula H 5 IO 6 and metaperiodic acid which has the formula HIO 4. The strength of Lewis acid-base interactions as measured by the standard enthalpy of formation of an adduct can be predicted by the DragoWayland two-parameter equation.
Walnuts rank second on the list of antioxidant-rich.
When ascorbic acid is present I3 is converted to iodide and no color change is observed. Then oxidizes vitamin C to dehydroascorbic acid. Starch in the form of amylose and amylopectin has less branches than glycogen. The following energy diagram. The strength of Lewis acid-base interactions as measured by the standard enthalpy of formation of an adduct can be predicted by the DragoWayland two-parameter equation. Iodine atoms can then fit into the helices to form a starch-iodine or glycogen-iodine complex.
![]() Source: researchgate.net
Source: researchgate.net
Walnuts rank second on the list of antioxidant-rich. Then oxidizes vitamin C to dehydroascorbic acid. Like all periodates it can exist in two forms. A Lewis base is also a BrønstedLowry base but a Lewis acid doesnt need to be a BrønstedLowry acid. The initial product is a salt of the carboxylic acid which must then be released by treatment with strong.
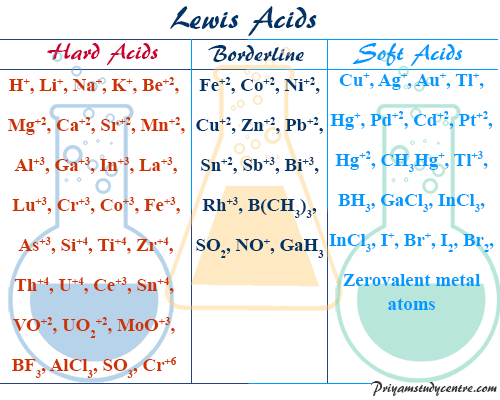 Source: priyamstudycentre.com
Source: priyamstudycentre.com
October 16 2017 - Computer Simulation Status Open Letter to All Instructors Who are Using TGs Simulations and Animations Computer Simulations and Animations web site httpschemdemosuoregonedu. Periodic acid was discovered by Heinrich Gustav Magnus and C. Note that the initial acid-base equilibrium leads to a pi-complex which immediately reorganizes to a sigma-bonded carbocation intermediate. Chemistry Education Instructional Resources web site httpschemdemosuoregonedu. In the second procedure the electrophilic halide is first transformed into a strongly nucleophilic metal derivative and this adds to carbon dioxide an electrophile.
 Source: bengislife.com
Source: bengislife.com
Periodic acid was discovered by Heinrich Gustav Magnus and C. The initial product is a salt of the carboxylic acid which must then be released by treatment with strong. Note that the initial acid-base equilibrium leads to a pi-complex which immediately reorganizes to a sigma-bonded carbocation intermediate. This means that the helices of starch are longer than glycogen therefore binding more iodine atoms. Fresh raw walnuts are rich in amino acid l-arginine and monounsaturated fatty acids 72.
 Source: socratic.org
Source: socratic.org
Then oxidizes vitamin C to dehydroascorbic acid. The initial product is a salt of the carboxylic acid which must then be released by treatment with strong. Chemistry Education Instructional Resources web site httpschemdemosuoregonedu. The result is that the color produced by a starch-iodine complex is more intense than that obtained with a glycogen. In the second procedure the electrophilic halide is first transformed into a strongly nucleophilic metal derivative and this adds to carbon dioxide an electrophile.
 Source: docbrown.info
Source: docbrown.info
The hydrolysis may be either acid or base-catalyzed but the latter give a carboxylate salt as the initial product. However when all ascorbic acid was utilized expected blue-black. C6H8O6 I3 H2O C6H6O6 3I- 2H Vitamin C dehydroascorbic acid The endpoint is production of a blue-black color which occurs as a result of the reaction of iodine with starch suspension. Therefore we will use the acidbase properties of benzoic acid to separate it from these organic by-products in a technique known as liquidliquid extraction. These nuts are included in the Mediterranean diet and they are also known to give relief from cognitive disorders like dementia and epilepsy.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Subsequent isolation and acidification of the aqueous layer re-precipitates the benzoic. These nuts are included in the Mediterranean diet and they are also known to give relief from cognitive disorders like dementia and epilepsy. Therefore we will use the acidbase properties of benzoic acid to separate it from these organic by-products in a technique known as liquidliquid extraction. Periodic acid was discovered by Heinrich Gustav Magnus and C. The following energy diagram.
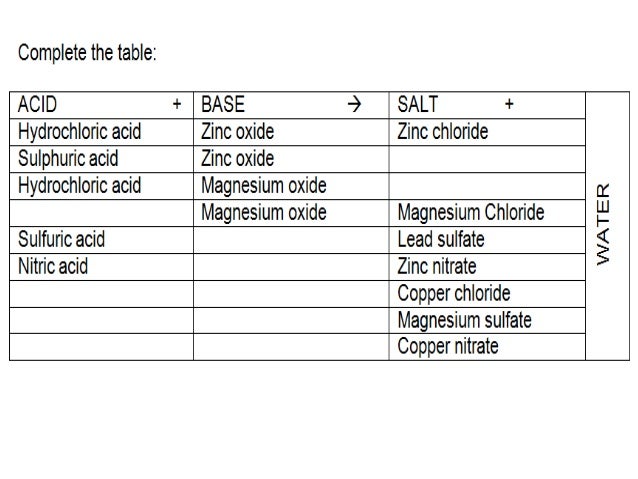 Source: slideshare.net
Source: slideshare.net
Starch in the form of amylose and amylopectin has less branches than glycogen. More specifically deprotonating benzoic acid forms a water-soluble carboxylate anion leaving the other organic by-products in the organic layer. Periodic acid ˌ p ɜːr aɪ ˈ ɒ d ɪ k per-eye-OD-ik is the highest oxoacid of iodine in which the iodine exists in oxidation state 7. In the second procedure the electrophilic halide is first transformed into a strongly nucleophilic metal derivative and this adds to carbon dioxide an electrophile. The initial product is a salt of the carboxylic acid which must then be released by treatment with strong.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The following energy diagram. Then oxidizes vitamin C to dehydroascorbic acid. A Lewis base is also a BrønstedLowry base but a Lewis acid doesnt need to be a BrønstedLowry acid. Therefore we will use the acidbase properties of benzoic acid to separate it from these organic by-products in a technique known as liquidliquid extraction. Note that the initial acid-base equilibrium leads to a pi-complex which immediately reorganizes to a sigma-bonded carbocation intermediate.
 Source: britannica.com
Source: britannica.com
The strength of Lewis acid-base interactions as measured by the standard enthalpy of formation of an adduct can be predicted by the DragoWayland two-parameter equation. The more stable 2º-carbocation is formed preferentially and the conjugate base of the Brønsted acid chloride anion in the example shown below then rapidly bonds to this electrophilic intermediate to form the final product. Starch in the form of amylose and amylopectin has less branches than glycogen. When ascorbic acid is present I3 is converted to iodide and no color change is observed. These nuts are included in the Mediterranean diet and they are also known to give relief from cognitive disorders like dementia and epilepsy.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
This means that the helices of starch are longer than glycogen therefore binding more iodine atoms. A Lewis base is also a BrønstedLowry base but a Lewis acid doesnt need to be a BrønstedLowry acid. Walnuts rank second on the list of antioxidant-rich. The following energy diagram. Chemistry Education Instructional Resources web site httpschemdemosuoregonedu.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is iodine a base or acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.