K2s acid or base
K2s Acid Or Base. A molecular compound that does not ionize in solution is considered a strong electrolyte. 1 mol g-formula-mass periodic table 1 mol 224 L for a gas at STP. Since it can not be a double displacement reaction in which two compounds react and cations and anions exchange their places to form new compounds. 1 atom S.
 K2s From franklychemistry.co.uk
K2s From franklychemistry.co.uk
Therefore the salicylic acid. Once 282 g of salicylic acid is reacted the reaction will stop even though there are 156 g of acetic anhydride present. 1 mol g-formula-mass periodic table 1 mol 224 L for a gas at STP. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Oxidation-reduction synthesis decomposition combustion two of these. A molecular compound that does not ionize in solution is considered a strong electrolyte.
Academiaedu is a platform for academics to share research papers.
3 grams of phosphorus. Since it can not be a double displacement reaction in which two compounds react and cations and anions exchange their places to form new compounds. Choose the statement below that is TRUE. Once 282 g of salicylic acid is reacted the reaction will stop even though there are 156 g of acetic anhydride present. Hg2Cl2 NH42CO3 Na2SO4 BaS All of these compounds are soluble in water. Academiaedu is a platform for academics to share research papers.
 Source: bengislife.com
Source: bengislife.com
The complete ionic. The complete ionic. Which of the following ionic compounds is soluble in water. Therefore the reaction is non of the above. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
 Source: youtube.com
Source: youtube.com
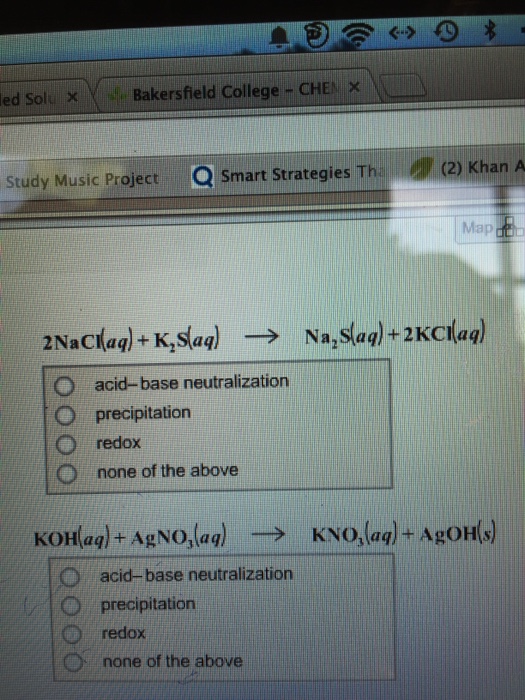
Since it can not be a double displacement reaction in which two compounds react and cations and anions exchange their places to form new compounds. Academiaedu is a platform for academics to share research papers. 2NaClaqK2Saq—Na2Saq2KClaq In this reaction both Na2S and KCl are soluble in water. 3 grams of phosphorus. The term weak electrolyte means that the.
3 grams of phosphorus. An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. The main differences between the titration problem on this worksheet and the first Titration and Buffer worksheet are that 1 this is a titration of a weak acid with a strong base and 2 the strong base produces 2 moles of OH-ions for every mole of base. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Therefore the reaction is non of the above.
 Source: studylib.net
Source: studylib.net
3 grams of phosphorus. Which of the following compounds is insoluble in water. Choose the statement below that is TRUE. 2NaClaqK2Saq—Na2Saq2KClaq In this reaction both Na2S and KCl are soluble in water. Once 282 g of salicylic acid is reacted the reaction will stop even though there are 156 g of acetic anhydride present.
 Source: youtube.com
Source: youtube.com
CoS and K2S LiNO3 and CaCO3 Hg2I2 and AgCl NaI andCuNO32. 2NaClaqK2Saq—Na2Saq2KClaq In this reaction both Na2S and KCl are soluble in water. How many molecules are there in 4. 3 grams of phosphorus. The complete ionic.
 Source: franklychemistry.co.uk
Source: franklychemistry.co.uk
K2S MgS CaS Al2S3 Cu2S. 1 mol g-formula-mass periodic table 1 mol 224 L for a gas at STP. The main differences between the titration problem on this worksheet and the first Titration and Buffer worksheet are that 1 this is a titration of a weak acid with a strong base and 2 the strong base produces 2 moles of OH-ions for every mole of base. The complete ionic. How many molecules are there in 4.

It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Acid-base two of the above. CoS and K2S LiNO3 and CaCO3 Hg2I2 and AgCl NaI andCuNO32. Which of the following ionic compounds is soluble in water. Once 282 g of salicylic acid is reacted the reaction will stop even though there are 156 g of acetic anhydride present.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Question 8 predict the product for the following reaction. Choose the statement below that is TRUE. Academiaedu is a platform for academics to share research papers. The complete ionic. Acid-base two of the above.
 Source: chemistrylearner.com
Source: chemistrylearner.com
The equation 2Ag2Os 4Ags O2g is an _____ reaction. The main differences between the titration problem on this worksheet and the first Titration and Buffer worksheet are that 1 this is a titration of a weak acid with a strong base and 2 the strong base produces 2 moles of OH-ions for every mole of base. Therefore the salicylic acid. Academiaedu is a platform for academics to share research papers. The equation 2Ag2Os 4Ags O2g is an _____ reaction.
 Source: chegg.com
Source: chegg.com
1 mol C4 H 6 O3 10209 g C4 H 6 O3 1 mol HOOCC6 H 4 OH 422 g HOOCC6H4OH Since more salicylic acid is required than is available it is the limiting reagent. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. CaOsCO2g—CaCO3s In this reaction CaO and CO2 combine to form calcium carbonate. 1 mol g-formula-mass periodic table 1 mol 224 L for a gas at STP. Since it can not be a double displacement reaction in which two compounds react and cations and anions exchange their places to form new compounds.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title k2s acid or base by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.