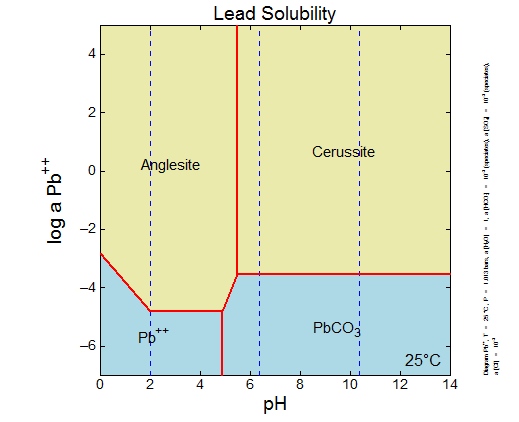
Lead carbonate solubility
Lead Carbonate Solubility. The diets were A control B control plus 1000 ppm iron as ferrous carbonate and C control plus 1000 ppm iron as ferrous sulfate monohydrateCalves were dosed orally on day 15 of the treatment period with 1 mCi of iron-59. From alkali metals only lithium forms insoluble carbonate. All alkaline earth metals forms insoluble. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon dioxide.
 1 Some Lead Compounds Their Molecular Weights Formula And Their Download Scientific Diagram From researchgate.net
1 Some Lead Compounds Their Molecular Weights Formula And Their Download Scientific Diagram From researchgate.net
Consequently calcium sulphate must be reacted upon chemically to cause a precipitate to form in the water where it can be. Precipitates of carbonate ion colours. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon dioxide. Li 2 CO 3 is a white solid precipitate compound. But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water. All alkaline earth metals forms insoluble.
All alkaline earth metals forms insoluble.
Consequently calcium sulphate must be reacted upon chemically to cause a precipitate to form in the water where it can be. Precipitates of carbonate ion colours. The diets were A control B control plus 1000 ppm iron as ferrous carbonate and C control plus 1000 ppm iron as ferrous sulfate monohydrateCalves were dosed orally on day 15 of the treatment period with 1 mCi of iron-59. Consequently calcium sulphate must be reacted upon chemically to cause a precipitate to form in the water where it can be. Li 2 CO 3 is a white solid precipitate compound. Twelve intact male Holstein calves averaging 90 kg and 12 wk of age were fed one of three dietary treatments for 28 days.
 Source: link.springer.com
Source: link.springer.com
Twelve intact male Holstein calves averaging 90 kg and 12 wk of age were fed one of three dietary treatments for 28 days. The diets were A control B control plus 1000 ppm iron as ferrous carbonate and C control plus 1000 ppm iron as ferrous sulfate monohydrateCalves were dosed orally on day 15 of the treatment period with 1 mCi of iron-59. High temperatures in the boiler water reduce the solubility of calcium sulphate and tend to make it precipitate out directly on the boiler metal as scale. Solubility of carbonates have a variation because there are soluble and insoluble carbonates. Twelve intact male Holstein calves averaging 90 kg and 12 wk of age were fed one of three dietary treatments for 28 days.
 Source: researchgate.net
Source: researchgate.net
Solubility of carbonates have a variation because there are soluble and insoluble carbonates. The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere pedosphere geosphere hydrosphere and atmosphere of the EarthCarbon is the main component of biological compounds as well as a major component of many minerals such as limestoneAlong with the nitrogen cycle and the water cycle the carbon cycle comprises a sequence of events that are key to. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon dioxide. High temperatures in the boiler water reduce the solubility of calcium sulphate and tend to make it precipitate out directly on the boiler metal as scale. But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon dioxide. Consequently calcium sulphate must be reacted upon chemically to cause a precipitate to form in the water where it can be. Solubility of carbonates have a variation because there are soluble and insoluble carbonates. High temperatures in the boiler water reduce the solubility of calcium sulphate and tend to make it precipitate out directly on the boiler metal as scale. Precipitates of carbonate ion colours.
 Source: en.wikipedia.org
Source: en.wikipedia.org
All alkaline earth metals forms insoluble. All alkaline earth metals forms insoluble. The diets were A control B control plus 1000 ppm iron as ferrous carbonate and C control plus 1000 ppm iron as ferrous sulfate monohydrateCalves were dosed orally on day 15 of the treatment period with 1 mCi of iron-59. Twelve intact male Holstein calves averaging 90 kg and 12 wk of age were fed one of three dietary treatments for 28 days. But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere pedosphere geosphere hydrosphere and atmosphere of the EarthCarbon is the main component of biological compounds as well as a major component of many minerals such as limestoneAlong with the nitrogen cycle and the water cycle the carbon cycle comprises a sequence of events that are key to. Precipitates of carbonate ion colours. But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water. Solubility of carbonates have a variation because there are soluble and insoluble carbonates. Consequently calcium sulphate must be reacted upon chemically to cause a precipitate to form in the water where it can be.
 Source: coalgeology.com
Source: coalgeology.com
Solubility of carbonates have a variation because there are soluble and insoluble carbonates. Precipitates of carbonate ion colours. But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water. From alkali metals only lithium forms insoluble carbonate. The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere pedosphere geosphere hydrosphere and atmosphere of the EarthCarbon is the main component of biological compounds as well as a major component of many minerals such as limestoneAlong with the nitrogen cycle and the water cycle the carbon cycle comprises a sequence of events that are key to.
 Source: researchgate.net
Source: researchgate.net
But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water. The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere pedosphere geosphere hydrosphere and atmosphere of the EarthCarbon is the main component of biological compounds as well as a major component of many minerals such as limestoneAlong with the nitrogen cycle and the water cycle the carbon cycle comprises a sequence of events that are key to. Consequently calcium sulphate must be reacted upon chemically to cause a precipitate to form in the water where it can be. Twelve intact male Holstein calves averaging 90 kg and 12 wk of age were fed one of three dietary treatments for 28 days. Precipitates of carbonate ion colours.
Source:
Consequently calcium sulphate must be reacted upon chemically to cause a precipitate to form in the water where it can be. From alkali metals only lithium forms insoluble carbonate. Precipitates of carbonate ion colours. Li 2 CO 3 is a white solid precipitate compound. Twelve intact male Holstein calves averaging 90 kg and 12 wk of age were fed one of three dietary treatments for 28 days.
 Source: youtube.com
Source: youtube.com
From alkali metals only lithium forms insoluble carbonate. High temperatures in the boiler water reduce the solubility of calcium sulphate and tend to make it precipitate out directly on the boiler metal as scale. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon dioxide. Solubility of carbonates have a variation because there are soluble and insoluble carbonates. Consequently calcium sulphate must be reacted upon chemically to cause a precipitate to form in the water where it can be.
 Source: sciencedirect.com
Source: sciencedirect.com
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere pedosphere geosphere hydrosphere and atmosphere of the EarthCarbon is the main component of biological compounds as well as a major component of many minerals such as limestoneAlong with the nitrogen cycle and the water cycle the carbon cycle comprises a sequence of events that are key to. Solubility of carbonates have a variation because there are soluble and insoluble carbonates. But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon dioxide. All alkaline earth metals forms insoluble.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lead carbonate solubility by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





