Lead ii chromate
Lead Ii Chromate. After the French chemist Louis Vauquelin discovered the new element chromium in 1797 lead chromate was synthesized in the laboratory and used as a pigment beginning in the second decade of the nineteenth. SnCN2 tin II cyanide 18. LeadIIchromate historical dye collection of the Technical University of Dresden Germany. BaOH2 barium hydroxide 22.

FeHSO32 iron II bisulfite 26. Lead II lead IV Bi3 Bi5 bismuthIII bismuthV Sn4 Sn2 tin IV tin II Sb3 Sb5 antimonyIII antimonyV Tc7 technitium Ta5 tantalum W6 tungsten Re7 rhenium Os4 osmium Ir4 iridium 87 88 89 H hydrogen Li lithium Be2 beryllium Na sodium Mg2 magnesium K potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C. 日本化学会編第4版 新実験化学講座 16巻 無機化合物 丸善1991年. Carbonate and thallate were absorbed to the greatest extent whereas absorption of sulfide chromate naphenate and octoate were absorbed at only 44-67 the rate of absorption for lead acetate Uptake of lead into the femurs of rats was highest for lead acetate intermediate for lead oxide and lowest. LeadIIchromate historical dye collection of the Technical University of Dresden Germany. SrNO32 strontium nitrate 27.
Lead reacts vigorously with fluorine F 2 at room temperature and chlorine Cl 2 when heated forming the corresponding leadII halides 8.
NH42CrO4 ammonium chromate 17. BaOH2 barium hydroxide 22. SnOH2 tin II hydroxide 28. It was formerly called plumbous iodide. NH42CrO4 ammonium chromate 17. LeadIIchromate historical dye collection of the Technical University of Dresden Germany.

2 2 X 1 chromate CrO 4 2 2 calcium Ca 2 2 mercury II mercuric Hg 2 2 cyanate OCN 1 cesium Cs 1 potassium K 1 cyanide CN 1 chromium II Cr 2 2 rubidium Rb 1 dichromate Cr 2 O 7 2 2 chromium III Cr 3 3 scandium III Sc 3 3 dihydrogen phosphate H 2 PO 4 1 cobalt II cobaltous Co 2 2 silver Ag 1 fluoride F 1 cobalt III. Lead reacts vigorously with fluorine F 2 at room temperature and chlorine Cl 2 when heated forming the corresponding leadII halides 8. SrNO32 strontium nitrate 27. BaOH2 barium hydroxide 22. CuClO42 copper II chlorate 25.

Carbonate and thallate were absorbed to the greatest extent whereas absorption of sulfide chromate naphenate and octoate were absorbed at only 44-67 the rate of absorption for lead acetate Uptake of lead into the femurs of rats was highest for lead acetate intermediate for lead oxide and lowest. After the French chemist Louis Vauquelin discovered the new element chromium in 1797 lead chromate was synthesized in the laboratory and used as a pigment beginning in the second decade of the nineteenth. 30 copper II chlorate CuClO32 31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42 35 silver cyanide AgCN 36 vanadium V nitride V3N5 37 strontium acetate SrC2H3O22 38 molybdenum sulfate. Carbonate and thallate were absorbed to the greatest extent whereas absorption of sulfide chromate naphenate and octoate were absorbed at only 44-67 the rate of absorption for lead acetate Uptake of lead into the femurs of rats was highest for lead acetate intermediate for lead oxide and lowest. CuClO42 copper II chlorate 25.
 Source: chemcraft.su
Source: chemcraft.su
LeadII is precipitated by chromate under acidic conditions. Pb 2 aq CrO 4 2 aq PbCrO 4 s yellow Reaction of lead with halogens. NH42CrO4 ammonium chromate 17. Lead reacts vigorously with fluorine F 2 at room temperature and chlorine Cl 2 when heated forming the corresponding leadII halides 8. 2 2 X 1 chromate CrO 4 2 2 calcium Ca 2 2 mercury II mercuric Hg 2 2 cyanate OCN 1 cesium Cs 1 potassium K 1 cyanide CN 1 chromium II Cr 2 2 rubidium Rb 1 dichromate Cr 2 O 7 2 2 chromium III Cr 3 3 scandium III Sc 3 3 dihydrogen phosphate H 2 PO 4 1 cobalt II cobaltous Co 2 2 silver Ag 1 fluoride F 1 cobalt III.

2 2 X 1 chromate CrO 4 2 2 calcium Ca 2 2 mercury II mercuric Hg 2 2 cyanate OCN 1 cesium Cs 1 potassium K 1 cyanide CN 1 chromium II Cr 2 2 rubidium Rb 1 dichromate Cr 2 O 7 2 2 chromium III Cr 3 3 scandium III Sc 3 3 dihydrogen phosphate H 2 PO 4 1 cobalt II cobaltous Co 2 2 silver Ag 1 fluoride F 1 cobalt III. SnCO32 tin IV carbonate 24. Mg3PO42 magnesium phosphate 20. BaOH2 barium hydroxide 22. NH42CrO4 ammonium chromate 17.

SnOH2 tin II hydroxide 28. CuClO42 copper II chlorate 25. FeHSO32 iron II bisulfite 26. LeadII is precipitated by chromate under acidic conditions. The compound currently has a few specialized applications such as the manufacture of solar cells and X-ray and gamma-ray detectors.

LeadII is precipitated by chromate under acidic conditions. Lead reacts vigorously with fluorine F 2 at room temperature and chlorine Cl 2 when heated forming the corresponding leadII halides 8. LeadII is precipitated by chromate under acidic conditions. 2 2 X 1 chromate CrO 4 2 2 calcium Ca 2 2 mercury II mercuric Hg 2 2 cyanate OCN 1 cesium Cs 1 potassium K 1 cyanide CN 1 chromium II Cr 2 2 rubidium Rb 1 dichromate Cr 2 O 7 2 2 chromium III Cr 3 3 scandium III Sc 3 3 dihydrogen phosphate H 2 PO 4 1 cobalt II cobaltous Co 2 2 silver Ag 1 fluoride F 1 cobalt III. CuClO42 copper II chlorate 25.
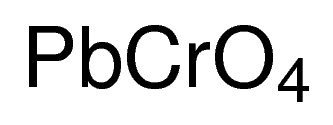 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It was formerly called plumbous iodide. NH42CrO4 ammonium chromate 17. HgCrO4 mercury II chromate 23. Lead II lead IV Bi3 Bi5 bismuthIII bismuthV Sn4 Sn2 tin IV tin II Sb3 Sb5 antimonyIII antimonyV Tc7 technitium Ta5 tantalum W6 tungsten Re7 rhenium Os4 osmium Ir4 iridium 87 88 89 H hydrogen Li lithium Be2 beryllium Na sodium Mg2 magnesium K potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C. LeadII iodide or lead iodide is a salt with the formula PbI 2.
 Source: en.wikipedia.org
Source: en.wikipedia.org
SnCN2 tin II cyanide 18. After the French chemist Louis Vauquelin discovered the new element chromium in 1797 lead chromate was synthesized in the laboratory and used as a pigment beginning in the second decade of the nineteenth. BaOH2 barium hydroxide 22. At room temperature it is a bright yellow odorless crystalline solid that becomes orange and red when heated. Pb 2 aq CrO 4 2 aq PbCrO 4 s yellow Reaction of lead with halogens.
 Source: en.wikipedia.org
Source: en.wikipedia.org
SnCN2 tin II cyanide 18. SnCN2 tin II cyanide 18. Pb s F 2 g PbF 2 s Pb s Cl 2 g PbCl 2 s LeadII is precipitated by chloride bromide and. It was formerly called plumbous iodide. Lead reacts vigorously with fluorine F 2 at room temperature and chlorine Cl 2 when heated forming the corresponding leadII halides 8.

Pb s F 2 g PbF 2 s Pb s Cl 2 g PbCl 2 s LeadII is precipitated by chloride bromide and. FeHSO32 iron II bisulfite 26. BaOH2 barium hydroxide 22. HgCrO4 mercury II chromate 23. PbSO42 lead IV sulfate 19.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lead ii chromate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.