Licl licl
Licl Licl. If both assertion and reason are correct and reason is the correct explanation of the assertion. Free essays homework help flashcards research papers book reports term papers history science politics. Lithium chloride LiCl or ClLi CID 433294 - structure chemical names physical and chemical properties classification patents literature biological activities. Al I 2 AlI 3 18.
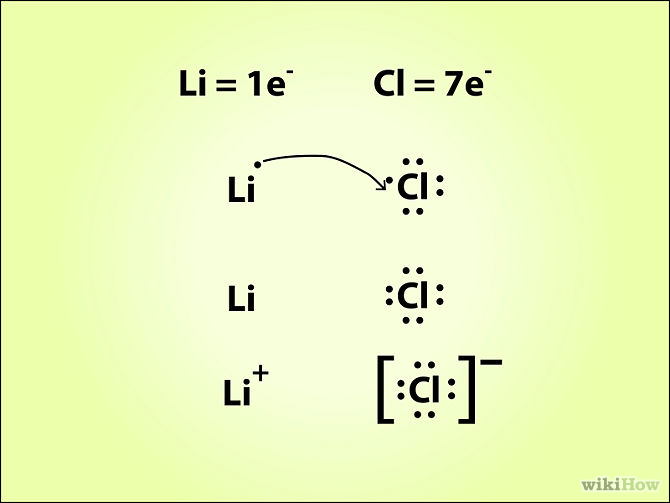
 Draw The Lewis Dot Structure Showing What Happens During The Reaction Lithium And Chlorine Produce Lithium Chloride Study Com From study.com
Draw The Lewis Dot Structure Showing What Happens During The Reaction Lithium And Chlorine Produce Lithium Chloride Study Com From study.com
1998 - 2 Marks Assertion. The most common ion formed by phosphorus has a charge of-3. NO O 2 NO 2 21. The gas thus formed is an effervescent carbon dioxide gas when calcium carbonate reacts with hydrochloric acid. Electronegativity difference between Li and Cl is too small. __ Li __ Cl.
These NIR bands were assigned to the first overtone of surface hydroxyls and interlayer hydroxyls of LiOH respectively.
It has both the highest oxygen to weight and oxygen to volume ratio of all practical perchlorate salts. This selectivity of saltbase differentiation rises by a factor of ca 20 when LiCl is taken at the high concentration of 35. 이를 이용하여 염화리튬습도계가 제작되어 시판되고 있다. C O 2 CO 25. HgO Hg O 2 17. If both assertion and reason are correct and reason is the correct explanation of the assertion.
 Source: study.com
Source: study.com
When a 1000 mL volume of 0100 M AgNO3 solution is mixed with a 1000 mL sample of 0200 M NaCl solution the temperature in the calorimeter rises to 2530C. Free essays homework help flashcards research papers book reports term papers history science politics. Al S Al 2 S 3 19. Though in all above presented examples the two electrolytes under separation had one common ion Na Li Cl or NH 4 the general formulation of the separation mechanism does not suggest the presence of a common ion as an essential prerequisite for the. The most common ion formed by phosphorus has a charge of-3.
 Source: socratic.org
Source: socratic.org
CH 4 O 2 CO 2 H 2 O 23. CH 4 O 2 CO 2 H 2 O 23. The solution contains a NaCl b HCl c LiCl d KCl. LiClO 4 LiCl 2 O 2. If both assertion and reason are correct and reason is the correct explanation of the assertion.
 Source: slideplayer.com
Source: slideplayer.com
1998 - 2 Marks Assertion. If both assertion and reason are correct and reason is the correct explanation of the assertion. Electronegativity difference between Li and Cl is too small. CaCO 3 2HCl CaCl 2 H 2 O CO 2. Chlorine now has 8 and is full.
 Source: slideplayer.com
Source: slideplayer.com
Al S Al 2 S 3 19. NO O 2 NO 2 21. Lithium chloride LiCl or ClLi CID 433294 - structure chemical names physical and chemical properties classification patents literature biological activities. These NIR bands were assigned to. These NIR bands were assigned to the first overtone of surface hydroxyls and interlayer hydroxyls of LiOH respectively.
 Source: youtube.com
Source: youtube.com
A LiLiF LiCl LiIPb-Sb cell with about 09 V open-circuit potential operating at 450 C had electroactive material costs of US100kWh and US100kW and a projected 25-year lifetime. 흡습용해성의 결정체의 고체이며 무색 또는 흰색을 띤다. 조해성이 있어 공기 중에서 수분을 흡수하여 녹는다. The drawback of the Li chemistry is higher cost. This selectivity of saltbase differentiation rises by a factor of ca 20 when LiCl is taken at the high concentration of 35.
 Source: researchgate.net
Source: researchgate.net
The most common ion formed by bromine has a charge of -1. Na H 2 O NaOH H 2 20. Enter the email address you signed up with and well email you a reset link. We would like to show you a description here but the site wont allow us. Electronegativity difference between Li and Cl is too small.
 Source: youtube.com
Source: youtube.com
Electronegativity difference between Li and Cl is too small. __ Li __ Cl 7 __ O 2. The eggshell contains calcium carbonate which on reaction with HCl liberates CO 2 gas which turn lime water to milky. Al S Al 2 S 3 19. Over 60 of the mass of the lithium perchlorate is released as oxygen.
 Source: youtube.com
Source: youtube.com
If assertion is correct but reason is incorrect. Enter the email address you signed up with and well email you a reset link. A LiLiF LiCl LiIPb-Sb cell with about 09 V open-circuit potential operating at 450 C had electroactive material costs of US100kWh and US100kW and a projected 25-year lifetime. This selectivity of saltbase differentiation rises by a factor of ca 20 when LiCl is taken at the high concentration of 35. Anhydrous LiOH showed two absorption bands at 7340 and 7171 cm1.
The carbon dioxide gas also acts as an. Over 60 of the mass of the lithium perchlorate is released as oxygen. The hydration behavior of LiOH LiOHH2O and LiCl was observed by near-infrared NIR spectroscopy. Electronegativity difference between Li and Cl is too small. LiCl is predominantly a covalent compound.

Electronegativity difference between Li and Cl is too small. PbO C Pb CO 2 24. Anhydrous LiOH showed two absorption bands at 7340 and 7171 cm1. Two solutions initially at 2460C are mixed in a coffee cup calorimeter Ccal 155 JC. Electronegativity difference between Li and Cl is too small.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title licl licl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.