Lithium chloride molar mass
Lithium Chloride Molar Mass. 86845 gmol Appearance White hygroscopic solid. Dont multiply the molar mass of a substance by the coefficient in the problem BEFORE using it in one of the steps above. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams. The atomic mass is useful in chemistry when it is paired with the mole concept.
 Molar Mass Of Licl Lithium Chloride Youtube From youtube.com
Molar Mass Of Licl Lithium Chloride Youtube From youtube.com
Here is a video which discusses how to calculate percent. Lithium is industrially produced mainly as lithium carbonate lithium hydroxide lithium chloride lithium bromide and butyl lithium. Dont multiply the molar mass of a substance by the coefficient in the problem BEFORE using it in one of the steps above. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Commercial manufacture of lithium aluminum hydride uses the original synthetic method. Modification of work by the Italian voiceFlickr.
Like lithium carbonate and lithium chloride it was used as treatment for bipolar disorder.
The same concept can be extended to ionic compounds and molecules. Dont multiply the molar mass of a substance by the coefficient in the problem BEFORE using it in one of the steps above. Like lithium carbonate and lithium chloride it was used as treatment for bipolar disorder. 86845 gmol Appearance White hygroscopic solid. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. Modification of work.
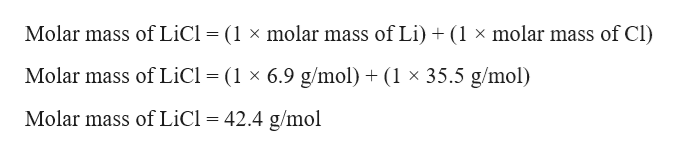 Source: bartleby.com
Source: bartleby.com
Thus it is essential to determine the rapid and accurate methods for separation. How many moles of KCI are required to prepare 150 L of 54 M KCl. In the 1940s as newer sedatives became available and when some heart patients died after using the salt substitute lithium chloride. Like lithium carbonate and lithium chloride it was used as treatment for bipolar disorder. Lithium is industrially produced mainly as lithium carbonate lithium hydroxide lithium chloride lithium bromide and butyl lithium.
 Source: youtube.com
Source: youtube.com
86845 gmol Appearance White hygroscopic solid. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Lithium chloride is a chemical compound with the formula Li ClThe salt is a typical ionic compound with certain covalent characters although the small size of the Li ion gives rise to properties not seen for other alkali metal chlorides such as extraordinary solubility in polar solvents 8305 g100 mL of water at 20 C and its hygroscopic properties. H 2g18g x 100 111 O 16g18g x 100 889. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams.
 Source: chegg.com
Source: chegg.com
One formula unit of sodium chloride. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. In the 1940s as newer sedatives became available and when some heart patients died after using the salt substitute lithium chloride. What is the molarity of a solution made by dissolving 394g of lithium chloride in enough water to make 159L of solution. The molar mass of H_2O is 18 gmol The hydrogens make up 2g since each mole of hydrogen is 1g The oxygen makes up 16g.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Addition of a diethyl ether solution of aluminum chloride to a slurry of lithium hydride. Lithium is industrially produced mainly as lithium carbonate lithium hydroxide lithium chloride lithium bromide and butyl lithium. H 2g18g x 100 111 O 16g18g x 100 889. Here is a video which discusses how to calculate percent. Modification of work by the Italian voiceFlickr.
 Source: youtube.com
Source: youtube.com
The stoichiometry 4 mol lithium hydride to 1 mol lithium aluminum hydride makes this an inherently expensive process even though high yields of pure product are obtained. Molar Mass of Frequently Calculated Chemicals. For example if the formula says 2H 2 O in the chemical equation DONT use 360 gmol use 180 gmol. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams. Thus it is essential to determine the rapid and accurate methods for separation.
 Source: youtube.com
Source: youtube.com
Percent composition can be calculated the chemical formula of a compound or it can be determined experimentally. Dont round off until the very last answer. Lithium chloride is a chemical compound with the formula Li ClThe salt is a typical ionic compound with certain covalent characters although the small size of the Li ion gives rise to properties not seen for other alkali metal chlorides such as extraordinary solubility in polar solvents 8305 g100 mL of water at 20 C and its hygroscopic properties. Commercial manufacture of lithium aluminum hydride uses the original synthetic method. Addition of a diethyl ether solution of aluminum chloride to a slurry of lithium hydride.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams. Modification of work by the Italian voiceFlickr. Thus it is essential to determine the rapid and accurate methods for separation. Like lithium carbonate and lithium chloride it was used as treatment for bipolar disorder. What is the molarity of a 24-liter solution containing 124.

The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. Dont round off until the very last answer. Lithium salts are psychoactive and. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. The stoichiometry 4 mol lithium hydride to 1 mol lithium aluminum hydride makes this an inherently expensive process even though high yields of pure product are obtained.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Modification of work. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Here is a video which discusses how to calculate percent. Commercial manufacture of lithium aluminum hydride uses the original synthetic method.
 Source: slideplayer.com
Source: slideplayer.com
How many grams of NaOH are in 500 mL of 0175 M NaOH solution. The same concept can be extended to ionic compounds and molecules. The molar mass of H_2O is 18 gmol The hydrogens make up 2g since each mole of hydrogen is 1g The oxygen makes up 16g. 86845 gmol Appearance White hygroscopic solid. Addition of a diethyl ether solution of aluminum chloride to a slurry of lithium hydride.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lithium chloride molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.