Mass of bacl2
Mass Of Bacl2. Close packed ABCB lighter flints CRTs vigorous wht La2O3 mild H2 LaOH3 mild H2 LaCl3 mild LaNO33 LaH2 LaH3 La2O3 LaCl3 malleable self-cleaning ovensCeO2 vigorous wht CeO2 mild H2 CeOH3 vigorous H2 CeCl3 mild CeNO33 CeH2 CeH3 Ce2O3 CeO2. Textmass of sulfate in the precipitate mass of sulfate in the unknown sample Finally using the mass of sulfate along with the initial mass of unknown used the percentage by mass of sulfate in the original. Grams 58443 5 292215 g. Grams mole molar mass.
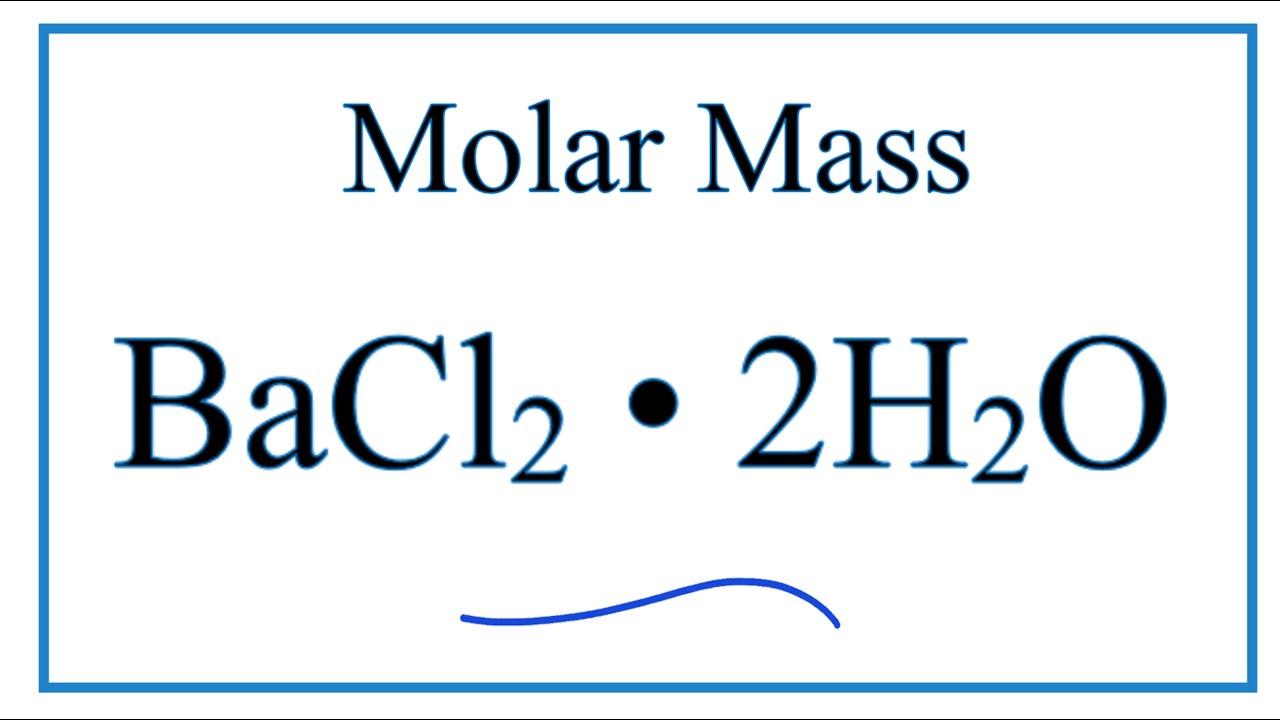 Molar Mass Molecular Weight Of Bacl2 2h2o Youtube From youtube.com
Molar Mass Molecular Weight Of Bacl2 2h2o Youtube From youtube.com
The mass of sulfate in the collected ceBaSO4 precipitate can be calculated via its percent composition. The student filters the BaSO4s rinses it and dries it until its mass is constant. Grams 58443 5 292215 g. If 9 g of water is decomposed we get 1 g of hydrogen and 8 g of oxygen. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Ammonia NH3 always.
Textmass of sulfate in the precipitate mass of sulfate in the unknown sample Finally using the mass of sulfate along with the initial mass of unknown used the percentage by mass of sulfate in the original.
Water - H 2 O. Mass fraction of a species is the mass of the species divided by the total mass of all species. If 9 g of water is decomposed we get 1 g of hydrogen and 8 g of oxygen. ML of a 600 M CaCl2. E338 H3Po4 PoOh3 Naval Jelly PhosphoricV Acid Orthophosphoric Acid White Phosphoric Acid H3PO4 Molar Mass H3PO4 Oxidation Number. Na3Po4 E339Iii E339Iii Na₃Po₄ Sodium Phosphate Sodium Orthophosphate Tribasic Sodium Phosphate Tertiary Sodium Phosphate Na3PO4 Molar Mass Na3PO4 Oxidation Number.
 Source: youtube.com
Source: youtube.com
This also yields the mass of sulfate in the original unknown since. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. The mass percent mm concentration of a solution is the mass of the solute divided by the solution mass multiplied by 100. A solution with 585 g sodium chloride dissolved in 5000 mL of water is 200 molar. 25 g TMAOH in 75 g.
 Source: youtube.com
Source: youtube.com
Which of the following scientific questions. The mass percent mm concentration of a solution is the mass of the solution divided by its volume multiplied by 100. Barium chloride is an inorganic compound with the formula Ba Cl 2It is one of the most common water-soluble salts of bariumLike most other barium salts it is white toxic and imparts a yellow-green coloration to a flame. If water is added to 100. So 25 wt TMAOH in MeOH could be.
 Source: youtube.com
Source: youtube.com
The mass percent mm concentration of a solution is the mass of the solute divided by the solution mass multiplied by 100. Bacl2 Barium Dichloride BaCl2 Molar Mass BaCl2 Oxidation Number Water - H 2 O Hoh Aqua Oh2 H₂O Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number. Trisodium Phosphate - Na 3 PO 4. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. 25 g TMAOH in 75 g.
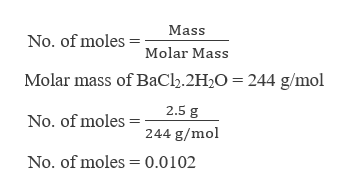 Source: bartleby.com
Source: bartleby.com
The student filters the BaSO4s rinses it and dries it until its mass is constant. ML of a 600 M CaCl2. Ammonia NH3 always. The student filters the BaSO4s rinses it and dries it until its mass is constant. So 25 wt TMAOH in MeOH could be.
 Source: slideplayer.com
Source: slideplayer.com
This also yields the mass of sulfate in the original unknown since. Grams mole molar mass. Water - H 2 O. The mass of sulfate in the collected ceBaSO4 precipitate can be calculated via its percent composition. ML of a 600 M CaCl2.
 Source: youtube.com
Source: youtube.com
The student filters the BaSO4s rinses it and dries it until its mass is constant. The mass percent mm concentration of a solution is the mass of the solution divided by its volume multiplied by 100. The mass percent mm concentration of a solution is the mass of the solute divided by the solution mass multiplied by 100. ML of a 600 M CaCl2. After completely dissolving the powder in 500mL of distilled water the student adds excess Na2SO4s which causes a precipitate of BaSO4s to form as represented by the equation above.
 Source: clutchprep.com
Source: clutchprep.com
Mass fraction of a species is the mass of the species divided by the total mass of all species. Which of the following scientific questions. The mass of sulfate in the collected ceBaSO4 precipitate can be calculated via its percent composition. Grams 58443 5 292215 g. The student filters the BaSO4s rinses it and dries it until its mass is constant.
 Source: youtube.com
Source: youtube.com
Ammonia NH3 always. Ammonia NH3 always. If water is added to 100. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. ML of a 600 M CaCl2.
 Source: youtube.com
Source: youtube.com
The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. ML of a 600 M CaCl2. If water is added to 100. BaCl2 solution BaSO4 white 3. A student obtains a 100g sample of a white powder labeled as BaCl2.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Textmass of sulfate in the precipitate mass of sulfate in the unknown sample Finally using the mass of sulfate along with the initial mass of unknown used the percentage by mass of sulfate in the original. Bacl2 Barium Dichloride BaCl2 Molar Mass BaCl2 Oxidation Number Water - H 2 O Hoh Aqua Oh2 H₂O Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number. After completely dissolving the powder in 500mL of distilled water the student adds excess Na2SO4s which causes a precipitate of BaSO4s to form as represented by the equation above. Grams mole molar mass.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title mass of bacl2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






