Mass of sodium hydroxide
Mass Of Sodium Hydroxide. Here the sodium hydroxide solution is not a primary standard and is taken in a burette and a known volume of the oxalic acid a standard solution is taken in the titration flask. For laboratory use sodium acetate is inexpensive and usually purchased instead of being synthesized. 2211 grams per cubic centimetre. How many oxygen atoms are in 812 g of calcium hydroxide.
 Solve This Q The Molar Mass Of The Solute Sodium Hydroxide Obtained From The Measurement Of The Osmotic Chemistry Solutions 12649921 Meritnation Com From meritnation.com
Solve This Q The Molar Mass Of The Solute Sodium Hydroxide Obtained From The Measurement Of The Osmotic Chemistry Solutions 12649921 Meritnation Com From meritnation.com
It is sometimes produced in a laboratory experiment by the reaction of acetic acid commonly in the 58 solution known as vinegar with sodium carbonate washing soda sodium bicarbonate baking soda or sodium hydroxide lye or caustic soda. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous CaCl 2 also yields this compound. In the case of strong acid. 106462 View Pricing Availability. Here the sodium hydroxide solution is not a primary standard and is taken in a burette and a known volume of the oxalic acid a standard solution is taken in the titration flask. What is the mass of 356 x 1025 formula units of copper II sulfate.
What is the mass of 356 x 1025 formula units of copper II sulfate.
What is the number of molecules present in 5000 g of diphosphorus pentoxide. In this example you are given the total mass and the percentage you want but are asked to find the amount of solute to add to the solution. The titration is carried out using phenolphthalein as an indicator. The molecular weight of sodium hydroxide is 40 gmol. Here the sodium hydroxide solution is not a primary standard and is taken in a burette and a known volume of the oxalic acid a standard solution is taken in the titration flask. Here the sodium hydroxide solution is taken in burette and a known volume 200 ml of the oxalic acid solution is taken in the titration flaskThe titration is carried out using phenolphthalein as indicator.
 Source: sahay.guru
Source: sahay.guru
The titration is carried out using phenolphthalein as an indicator. Sodium hydroxide is deliquescent absorbs moisture from the atmosphere solid. In the case of strong acid. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound. Here the sodium hydroxide solution is not a primary standard and is taken in a burette and a known volume of the oxalic acid a standard solution is taken in the titration flask.
 Source: meritnation.com
Source: meritnation.com
Here the sodium hydroxide solution is taken in burette and a known volume 200 ml of the oxalic acid solution is taken in the titration flaskThe titration is carried out using phenolphthalein as indicator. The total mass of the compound is the amount of sodium hydroxide plus the amount of water. In an acid-base titration the amount of acid becomes chemically equivalent to the amount of base present in the end. It is not. For laboratory use sodium acetate is inexpensive and usually purchased instead of being synthesized.

Include all units and account for. Sodium hydroxide solution of about 02 M is prepared in order to be used in Exp 12B. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous CaCl 2 also yields this compound. For laboratory use sodium acetate is inexpensive and usually purchased instead of being synthesized. In this example you are given the total mass and the percentage you want but are asked to find the amount of solute to add to the solution.
 Source: study.com
Source: study.com
Sodium hydroxide MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. Show all work utilizing dimensional analysis wherever possible. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound. Visit BYJUS for more information. 2211 grams per cubic centimetre.
 Source: chegg.com
Source: chegg.com
What is the number of molecules present in 5000 g of diphosphorus pentoxide. Soda caustic CAS. Include all units and account for. In an acid-base titration the amount of acid becomes chemically equivalent to the amount of base present in the end. 106462 View Pricing Availability.
 Source: bartleby.com
Source: bartleby.com
In an acid-base titration the amount of acid becomes chemically equivalent to the amount of base present in the end. Show all work utilizing dimensional analysis wherever possible. It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH. CaOH 2 has a hexagonal crystal structure.
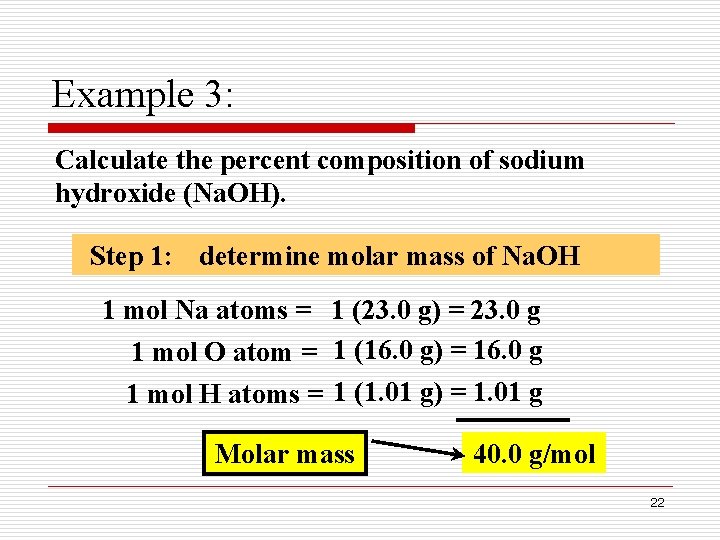 Source: slidetodoc.com
Source: slidetodoc.com
It is sometimes produced in a laboratory experiment by the reaction of acetic acid commonly in the 58 solution known as vinegar with sodium carbonate washing soda sodium bicarbonate baking soda or sodium hydroxide lye or caustic soda. 106462 View Pricing Availability. What is the mass of 356 x 1025 formula units of copper II sulfate. Colourless to pink Alkali in burette Procedure. Visit BYJUS for more information.
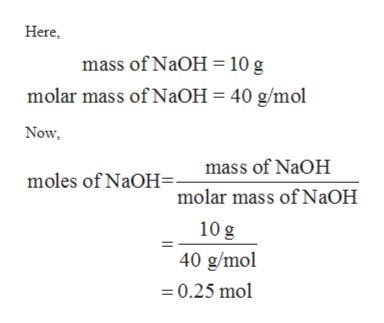 Source: bartleby.com
Source: bartleby.com
Take a burette and wash it with water. Sodium hydroxide MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. Sodium hydroxide solution of about 02 M is prepared in order to be used in Exp 12B. What is the mass of 356 x 1025 formula units of copper II sulfate. In this example you are given the total mass and the percentage you want but are asked to find the amount of solute to add to the solution.
 Source: youtube.com
Source: youtube.com
CaOH 2 has a hexagonal crystal structure. It is sometimes produced in a laboratory experiment by the reaction of acetic acid commonly in the 58 solution known as vinegar with sodium carbonate washing soda sodium bicarbonate baking soda or sodium hydroxide lye or caustic soda. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound. Download Product Safety Card. In an acid-base titration the amount of acid becomes chemically equivalent to the amount of base present in the end.
 Source: youtube.com
Source: youtube.com
In the case of strong acid. 40 gmol Chemical Formula. Sodium hydroxide MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. How many oxygen atoms are in 812 g of calcium hydroxide. The molecular weight of sodium hydroxide is 40 gmol.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title mass of sodium hydroxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.