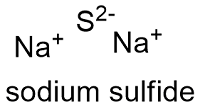Mercury 2 oxide decomposition formula
Mercury 2 Oxide Decomposition Formula. An oxide ˈ ɒ k s aɪ d is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. Sodium sulfate 2Na SO 4 2- Na 2 SO 4. Most of the Earths crust consists of solid oxides the result of elements being oxidized by the. See Butyl mercaptan.
 Oxygen Discovery Symbol Properties Uses Facts Britannica From britannica.com
Oxygen Discovery Symbol Properties Uses Facts Britannica From britannica.com
2 Determine the moles then mass of O. Sodium sulfate 2Na SO 4 2- Na 2 SO 4. Preparation of Silver Nitrate. Mercury Certified ACS Revision Date 17-Jan-2018 Hazardous Decomposition ProductsMercury oxide Highly toxic fumes Hazardous Polymerization Hazardous polymerization does not occur. STEL 1 ppm5 ppm Butanethiol. A P b M o O 4 b P b M o 2 O c P b 2 M o O 2 d P b M o O 3 e P b M o O 2 View Answer Calculate the mass percent composition for the compound with 125 grams of K 172 grams of Mn and 20.
Nitrate mercurique French MercuryII nitrate 12 Nitric acid mercuryII salt.
2 Determine the moles then mass of O. Potassium carbonate K 2. 7637-07-2 C1 C3 Bromine. From the results of the experiment determine the molecular formula of this oxide of mercury Solution. See Butyl mercaptan. Sodium sulfate 2Na SO 4 2- Na 2 SO 4.
 Source: slideplayer.com
Source: slideplayer.com
AgNO3 aq NaCl aq AgCl s NaNO3 aq Ag aq C1 - aq AgCl s ionic equation Colour of Salt. ZnCO 3—– ZnO CO 2. Sodium sulfate Na 2 SO 4. Insoluble salts can be made by double decomposition. 10 Boron trifluoride.
 Source: youtube.com
Source: youtube.com
Most of the Earths crust consists of solid oxides the result of elements being oxidized by the. See 29 CFR 19101051. ZnCO 3—– ZnO CO 2. Salt or metal. Nitric acid mercury2 salt 21 Citrine ointment.
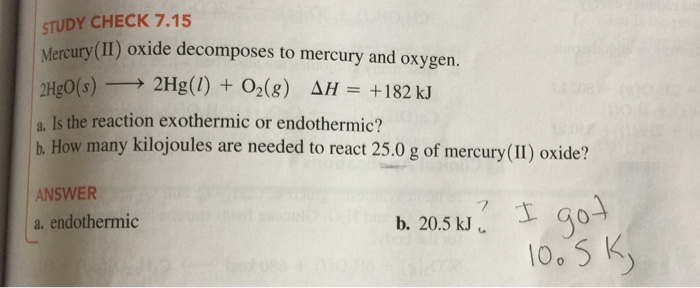
A P b M o O 4 b P b M o 2 O c P b 2 M o O 2 d P b M o O 3 e P b M o O 2 View Answer Calculate the mass percent composition for the compound with 125 grams of K 172 grams of Mn and 20. Sodium sulfate 2Na SO 4 2- Na 2 SO 4. Mass of test tube oxide of mercury 1761 g Volume of oxygen collected at RTP 120. Total dust 15 Boron tribromide. Nitrate mercurique French MercuryII nitrate 12 Nitric acid mercuryII salt.
 Source: youtube.com
Source: youtube.com
Toxicological information Acute Toxicity. B the chemical formula of the compound appears after the arrow. Nitrate mercurique French MercuryII nitrate 12 Nitric acid mercuryII salt. Sodium sulfate 2Na SO 4 2- Na 2 SO 4. 29 CFR 191019l 106-99-0.

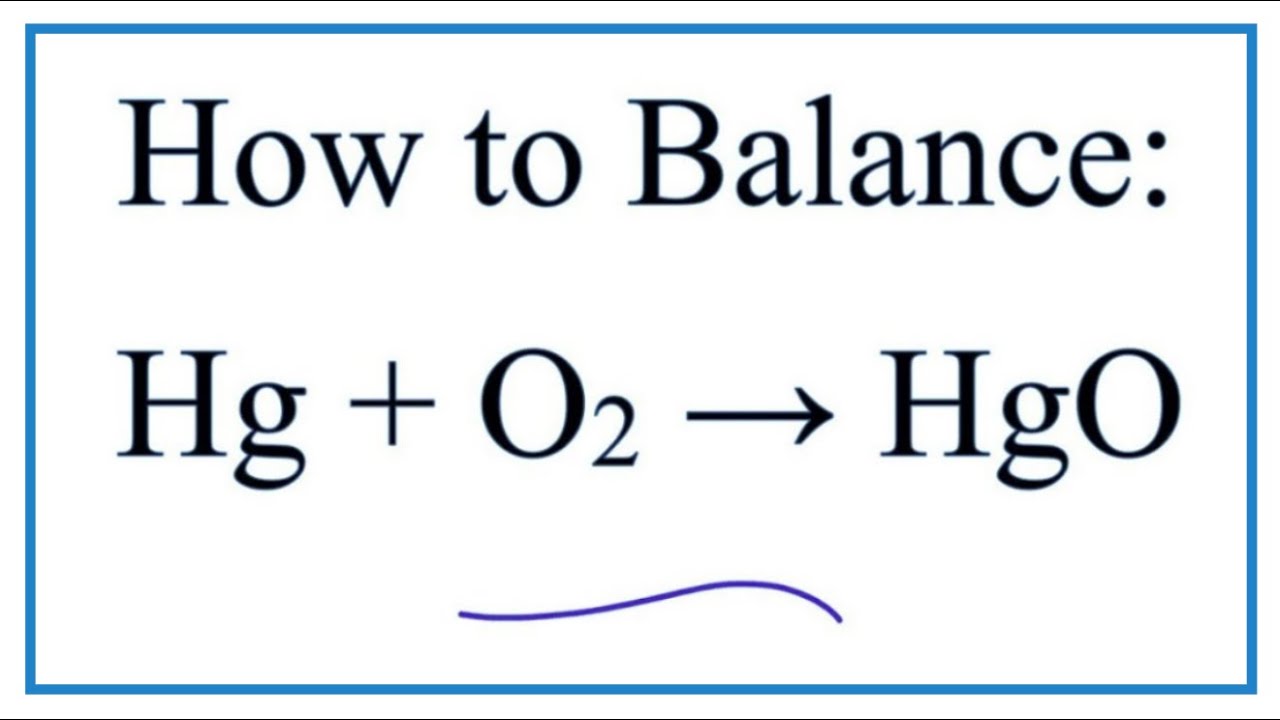
An example of a decomposition reaction is mercury II oxide decomposing into mercury metal and oxygen gas in the presence of heat. Nitrate mercurique French MercuryII nitrate 12 Nitric acid mercuryII salt. Thermal decomposition is used to obtain mercury metal from its oxide that is mercuric oxide Thermal decomposition is used to obtain zinc oxide from its carbonate ore. Mercury Certified ACS Revision Date 17-Jan-2018 Hazardous Decomposition ProductsMercury oxide Highly toxic fumes Hazardous Polymerization Hazardous polymerization does not occur. Hazardous Reactions None under normal processing.
 Source: britannica.com
Source: britannica.com
Acid Metal Oxide Salt H2O. Thermal decomposition is used to obtain mercury metal from its oxide that is mercuric oxide Thermal decomposition is used to obtain zinc oxide from its carbonate ore. Mass of test tube oxide of mercury 1761 g Volume of oxygen collected at RTP 120. Nitric acid mercury2 salt. ZnCO 3—– ZnO CO 2.
 Source: youtube.com
Source: youtube.com
This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. Total dust 15 Boron tribromide. Most of the Earths crust consists of solid oxides the result of elements being oxidized by the. This involves mixing a solution that contains its positive ions with another solution that contains its negative ions. Thermal decomposition is used to obtain mercury metal from its oxide that is mercuric oxide Thermal decomposition is used to obtain zinc oxide from its carbonate ore.
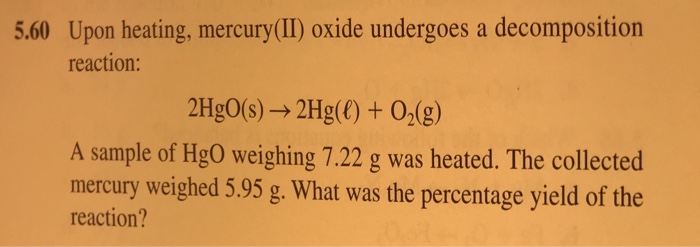
07 Bromine pentafluoride. An oxide ˈ ɒ k s aɪ d is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. Potassium carbonate 2K CO 3 2- K 2 CO 3. Millons reagent for the detection of Tyrosine. A P b M o O 4 b P b M o 2 O c P b 2 M o O 2 d P b M o O 3 e P b M o O 2 View Answer Calculate the mass percent composition for the compound with 125 grams of K 172 grams of Mn and 20.
 Source: slideplayer.com
Source: slideplayer.com
STEL 1 ppm5 ppm Butanethiol. 07 Bromine pentafluoride. See Butyl mercaptan. Mercury II oxide a red solid decomposes when heated to produce mercury and oxygen gas. 1 Mass of oxide.
 Source: youtube.com
Source: youtube.com
2 Determine the moles then mass of O. Potassium carbonate K 2. Toxicological information Acute Toxicity. Mass of test tube oxide of mercury 1761 g Volume of oxygen collected at RTP 120. STEL 1 ppm5 ppm Butanethiol.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title mercury 2 oxide decomposition formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.