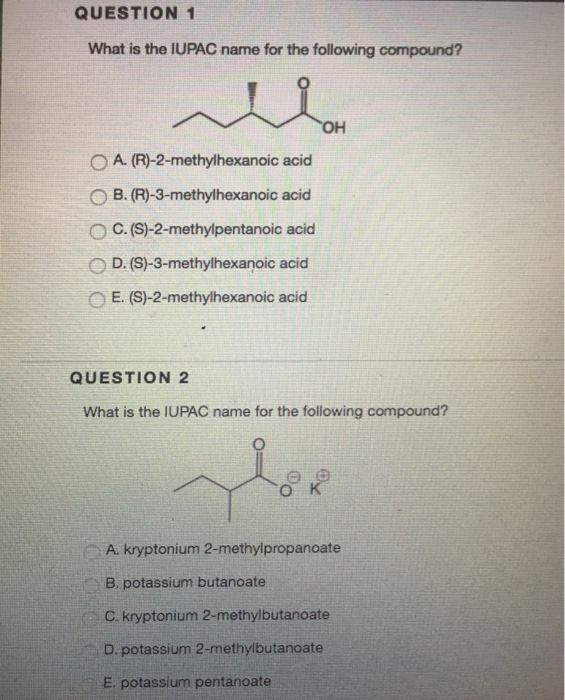
Mercury i chloride formula
Mercury I Chloride Formula. Silver chloride is a white crystalline chemical compound with the formula AgCl. Co 2SO43 cobalt III sulfate 60. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. Potassium bromide KBr 62.
 How To Write The Formula For Mercury Ii Chloride Youtube From youtube.com
How To Write The Formula For Mercury Ii Chloride Youtube From youtube.com
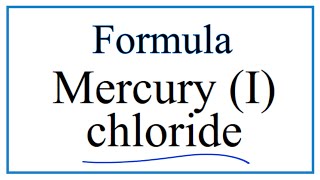
Mg3N2 magnesium nitride 49. Sulfur dioxide SO2_ 2. Hg2Cl2 mercury I chloride 57. You should complete this by Sunday. Give the formula for the following. Silver chloride is a chemical compound with the chemical formula Ag ClThis white crystalline solid is well known for its low solubility in water this behavior being reminiscent of the chlorides of Tl and Pb 2Upon illumination or heating silver chloride converts to silver and chlorine which is signaled by grey to black or purplish coloration to some samples.
Mercury has been used in manufacturing as well as in dental and medical equipment fertilizers and pesticides.
KCIO potassium hypochlorite 48. Silver chloride is an. Sulfur dioxide SO2_ 2. KCIO potassium hypochlorite 48. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. Silver chloride in the test tube quickly turns purplish especially in a sunny laboratory because the silver chloride is split up into silver and chlorine.
 Source: youtube.com
Source: youtube.com
Melting Point C Physical Form. Silver chloride is prepared when sodium chloride is added to silver nitrate solution a white precipitate of silver chloride occurs. NaMnO4 sodium permanganate 50. Melting Point C Physical Form. KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds.
 Source: youtube.com
Source: youtube.com
Mercury has been used in manufacturing as well as in dental and medical equipment fertilizers and pesticides. Silver chloride is prepared when sodium chloride is added to silver nitrate solution a white precipitate of silver chloride occurs. Complete these in lab and on your own time for practice. NaMnO4 sodium permanganate 50. Mg3N2 magnesium nitride 49.
 Source: youtube.com
Source: youtube.com
Sodium thiosulfate Na2S2O3_ 3. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. NaMnO4 sodium permanganate 50. You should complete this by Sunday.
 Source: youtube.com
Source: youtube.com
ZnCH3COO2 zinc acetate 58. ZnCH3COO2 zinc acetate 58. Mercury is a naturally occurring trace metalloid element and known neurotoxin with atomic symbol Hg atomic number 80 and atomic weight 20059. Mg3N2 magnesium nitride 49. Hg2Cl2 mercury I chloride 57.
 Source: youtube.com
Source: youtube.com
KCIO potassium hypochlorite 48. Silver chloride is an. Give the formula for the following. Mercury has been used in manufacturing as well as in dental and medical equipment fertilizers and pesticides. Silver chloride in the test tube quickly turns purplish especially in a sunny laboratory because the silver chloride is split up into silver and chlorine.
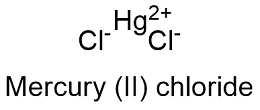 Source: softschools.com
Source: softschools.com
KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds. Complete these in lab and on your own time for practice. Mg3N2 magnesium nitride 49. Silver chloride in the test tube quickly turns purplish especially in a sunny laboratory because the silver chloride is split up into silver and chlorine. KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds.
 Source: fishersci.se
Source: fishersci.se
Sulfur dioxide SO2_ 2. It is characterized as a heavy silvery-white metallic liquid at room temperature that is odorless. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. Silver chloride is prepared when sodium chloride is added to silver nitrate solution a white precipitate of silver chloride occurs. Barium sulfide BaS 72.

Melting Point C Physical Form. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. K2SO4 potassium sulfate 59. Melting Point C Physical Form. ZnCH3COO2 zinc acetate 58.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Melting Point C Physical Form. Complete these in lab and on your own time for practice. Silver chloride in the test tube quickly turns purplish especially in a sunny laboratory because the silver chloride is split up into silver and chlorine. Give the formula for the following. KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds.

Sodium chloride is the salt most responsible. Mg3N2 magnesium nitride 49. Sulfur dioxide SO2_ 2. Barium sulfide BaS 72. KCIO potassium hypochlorite 48.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title mercury i chloride formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.