Mercury oxide water solubility
Mercury Oxide Water Solubility. 136 gcm-3 at 20C. Above pH 11 solubility also increases. Evacuate personnel to safe areas. MercuryII chloride or mercuric chloride historically also known as corrosive sublimate is the chemical compound of mercury and chlorine with the formula HgCl 2It is white crystalline solid and is a laboratory reagent and a molecular compound that is very toxic to humans.
 Mercury Ii Oxide Wikipedia From en.wikipedia.org
Mercury Ii Oxide Wikipedia From en.wikipedia.org
Melting point of mercury 389 C. Hexavalent chromium CrVI is the most oxidized form a potentially dangerous substance due to its high solubility and mobility which allows it to infiltrate biological membranes and pollute soil. Above pH 11 solubility also increases. 136 gcm-3 at 20C. Mercury has been used in manufacturing as well as in dental and medical equipment fertilizers and pesticides. Magnesium hydroxide forms in the presence of water.
Once used as a treatment for syphilis it is no longer used for medicinal purposes because of mercury toxicity and the.
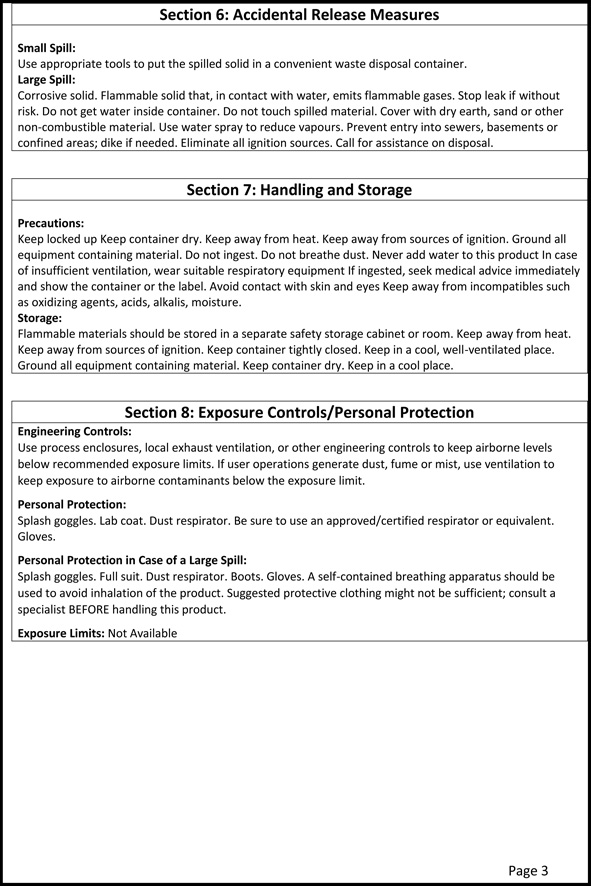
Hexavalent chromium CrVI is the most oxidized form a potentially dangerous substance due to its high solubility and mobility which allows it to infiltrate biological membranes and pollute soil. Accidental release measures Personal Precautions Wear self-contained breathing apparatus and protective suit. Mercury is a naturally occurring trace metalloid element and known neurotoxin with atomic symbol Hg atomic number 80 and atomic weight 20059. Zinc chloride ZnCl 2 4320 gL and zinc oxide ZnO or zinc vitriol ZnSO 4. 7H 2 O 580 gL. Zinc dissolves in water as ZnOH aq or Zn 2 aq.
 Source: scielo.br
Source: scielo.br
Electronegativity according to Pauling. ChromiumIII species are less soluble and relatively immobile as a result of their adsorption to clays and oxide minerals below pH 5 while low solubility above pH 5 is as a result of CrOH 3 S. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. 7H 2 O 580 gL. Magnesium oxide Mg O or magnesia is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium see also oxide.
 Source: sciencedirect.com
Source: sciencedirect.com
Mercury oxide Highly toxic fumes Protective Equipment and Precautions for Firefighters As in any fire wear self-contained breathing apparatus pressure-demand MSHANIOSH approved or equivalent and full protective gear. MercuryII chloride or mercuric chloride historically also known as corrosive sublimate is the chemical compound of mercury and chlorine with the formula HgCl 2It is white crystalline solid and is a laboratory reagent and a molecular compound that is very toxic to humans. Above pH 11 solubility also increases. Hexavalent chromium CrVI is the most oxidized form a potentially dangerous substance due to its high solubility and mobility which allows it to infiltrate biological membranes and pollute soil. Mercury oxide Highly toxic fumes Protective Equipment and Precautions for Firefighters As in any fire wear self-contained breathing apparatus pressure-demand MSHANIOSH approved or equivalent and full protective gear.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Electronegativity according to Pauling. Magnesium hydroxide forms in the presence of water. Accidental release measures Personal Precautions Wear self-contained breathing apparatus and protective suit. Melting point of mercury 389 C. It has an empirical formula of The template Magnesium is being considered for deletion Mg O and consists of a lattice of Mg 2 ions and O 2 ions held together by ionic bonding.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Hexavalent chromium CrVI is the most oxidized form a potentially dangerous substance due to its high solubility and mobility which allows it to infiltrate biological membranes and pollute soil. Solubility increases with increasing acidity. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. Magnesium oxide Mg O or magnesia is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium see also oxide. Substance Formula Melting point C Boiling temperature C Density 25C gcm 3 Liquid density Melting point gcm 3 Water solubility 25C 1 g100g H 2 O Comments Aluminium.
 Source: cdnsciencepub.com
Source: cdnsciencepub.com
54 Likes 13 Comments - UCLA VA Physiatry Residency uclava_pmrresidency on Instagram. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. Melting point of mercury 389 C. 136 gcm-3 at 20C. Evacuate personnel to safe areas.
 Source: 123rf.com
Source: 123rf.com
Examples of solubility of zinc compounds are. 136 gcm-3 at 20C. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. Mercury has been used in manufacturing as well as in dental and medical equipment fertilizers and pesticides. Hexavalent chromium CrVI is the most oxidized form a potentially dangerous substance due to its high solubility and mobility which allows it to infiltrate biological membranes and pollute soil.
 Source: cdnsciencepub.com
Source: cdnsciencepub.com
MercuryII chloride or mercuric chloride historically also known as corrosive sublimate is the chemical compound of mercury and chlorine with the formula HgCl 2It is white crystalline solid and is a laboratory reagent and a molecular compound that is very toxic to humans. Mercury is a naturally occurring trace metalloid element and known neurotoxin with atomic symbol Hg atomic number 80 and atomic weight 20059. Substance Formula Melting point C Boiling temperature C Density 25C gcm 3 Liquid density Melting point gcm 3 Water solubility 25C 1 g100g H 2 O Comments Aluminium. Why is zinc present. Examples of solubility of zinc compounds are.
 Source: sciencedirect.com
Source: sciencedirect.com
Melting point of mercury 389 C. Solubility increases with increasing acidity. Magnesium oxide Mg O or magnesia is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium see also oxide. Examples of solubility of zinc compounds are. Above pH 11 solubility also increases.

It has an empirical formula of The template Magnesium is being considered for deletion Mg O and consists of a lattice of Mg 2 ions and O 2 ions held together by ionic bonding. Van Der Waals radius. Zinc dissolves in water as ZnOH aq or Zn 2 aq. Accidental release measures Personal Precautions Wear self-contained breathing apparatus and protective suit. Substance Formula Melting point C Boiling temperature C Density 25C gcm 3 Liquid density Melting point gcm 3 Water solubility 25C 1 g100g H 2 O Comments Aluminium.
 Source: mdpi.com
Source: mdpi.com
136 gcm-3 at 20C. Above pH 11 solubility also increases. Accidental release measures Personal Precautions Wear self-contained breathing apparatus and protective suit. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. ChromiumIII species are less soluble and relatively immobile as a result of their adsorption to clays and oxide minerals below pH 5 while low solubility above pH 5 is as a result of CrOH 3 S.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title mercury oxide water solubility by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.