Methanol molar mass
Methanol Molar Mass. 144105 gmol 1 Appearance white or colorless crystalline powder Odor. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Once you have calculated the desired mass you need to weigh it out. 16 x 3 48.
 What Is Methanol Methanol Structure Density Molar Mass Study Com From study.com
What Is Methanol Methanol Structure Density Molar Mass Study Com From study.com
At 32 kgkmol it has the same molecular mass as oxygen and so from a. Methanols density is higher than that of gasoline despite its low molecular mass. What is the molar mass of sodium carbonate Na2CO3. In the NaOH. Therefore the molar mass of Na2CO3 is 106 gmol. Atomic mass of Oxygen 1600.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
16 x 3 48. Dilute the compound with. The concept of an alcohol being inside the membrane thus does not applytopologically methanols are always. This is due to the methanol molecule being polar due to its OH group causing hydrogen bonding. 6269 g100 mL 0 C 6278 g100 mL 15 C 6287 g100 mL 30 C 7111 g100 mL 100 C Solubility. Always clean the balance of any powder before continuing to make the solution.
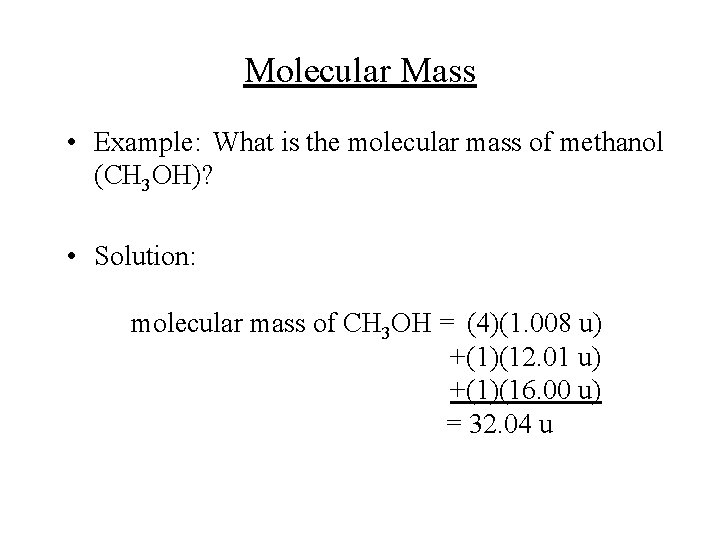 Source: slidetodoc.com
Source: slidetodoc.com
683 K Solubility in water. Atomic mass of Oxygen 1600. 21950 gmol dihydrate 18348 gmol anhydrous Appearance White solid all forms Density. We can also use molecular weight calculator for finding molar mass of a. Soluble in liquid ammonia pyridine.
 Source: researchgate.net
Source: researchgate.net
43 g100 mL 20 C dihydrate Solubility. 6269 g100 mL 0 C 6278 g100 mL 15 C 6287 g100 mL 30 C 7111 g100 mL 100 C Solubility. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Weigh out the necessary amount of compound in grams and set it aside. At 32 kgkmol it has the same molecular mass as oxygen and so from a.
 Source: study.com
Source: study.com
The concept of an alcohol being inside the membrane thus does not applytopologically methanols are always. Dilute the compound with. If molecular formula calculator add up the total value which is 12 46 48 106. Weigh out 25 g of NaCl. Well add those numbers together along with the unit grams per mole for finding molar mass.
 Source: youtube.com
Source: youtube.com
16 x 3 48. Decomposes at 237 C 459 F. 230 x 2 46. Therefore the molar mass of Na2CO3 is 106 gmol. Molar mass of Carbon Monoxide 2801.
 Source: youtube.com
Source: youtube.com
This is due to the methanol molecule being polar due to its OH group causing hydrogen bonding. If molecular formula calculator add up the total value which is 12 46 48 106. 16 x 3 48. We can also use molecular weight calculator for finding molar mass of a. Dilute the compound with.
 Source: thermopedia.com
Source: thermopedia.com
Weigh out the necessary amount of compound in grams and set it aside. This way we can calculate the molar mass of a compound or one-carbon compound. 230 x 2 46. 822 g100 g 15 C 755 g100 g 662 C. Always clean the balance of any powder before continuing to make the solution.
 Source: researchgate.net
Source: researchgate.net
16 x 3 48. 683 K Solubility in water. Weigh out the necessary amount of compound in grams and set it aside. Decomposes Solubility in water. 6269 g100 mL 0 C 6278 g100 mL 15 C 6287 g100 mL 30 C 7111 g100 mL 100 C Solubility.
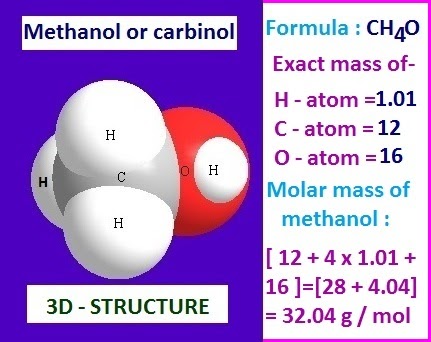 Source: chemizi.blogspot.com
Source: chemizi.blogspot.com
The above observation is also able to explain why no hydrogen bonds are formed between methanol and the lipids. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. This way we can calculate the molar mass of a compound or one-carbon compound. 120 x 1 12. 822 g100 g 15 C 755 g100 g 662 C.
 Source: youtube.com
Source: youtube.com
410 C 770 F. Well add those numbers together along with the unit grams per mole for finding molar mass. This is due to the methanol molecule being polar due to its OH group causing hydrogen bonding. 510 K dihydrate loses water at 100 C Boiling point. This way we can calculate the molar mass of a compound or one-carbon compound.
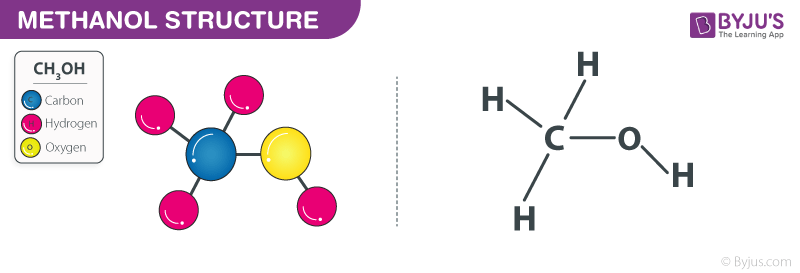 Source: byjus.com
Source: byjus.com
15 g100 mL methanol Magnetic susceptibility χ 101010 6 cm 3 mol 2. Decomposes Solubility in water. This way we can calculate the molar mass of a compound or one-carbon compound. 21950 gmol dihydrate 18348 gmol anhydrous Appearance White solid all forms Density. The above observation is also able to explain why no hydrogen bonds are formed between methanol and the lipids.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title methanol molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.