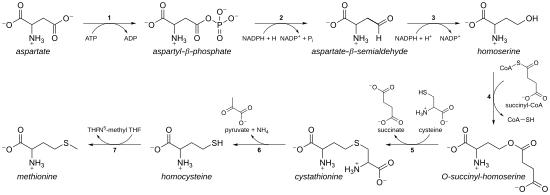
Methylene chloride chemical structure
Methylene Chloride Chemical Structure. When the shaking stops the oxygen comes out of the solution and it goes back to colorless. Methylene chloride is primarily used as a solvent in paint removers but is also used in aerosol formulations as a solvent in the manufacture of pharmaceuticals as a degreasing agent in. This is because owing to the structure the carbon is especially acidic and can easily be deprotonated to form a methylene group. In systematic chemical nomenclature alkynes are named with the Greek prefix system without any additional letters.
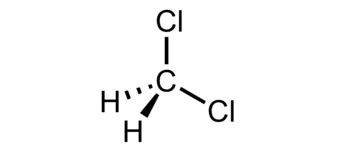 Methylene Chloride Dichloromethane Oehha From oehha.ca.gov
Methylene Chloride Dichloromethane Oehha From oehha.ca.gov
This is a reversible redox reaction. When the shaking stops the oxygen comes out of the solution and it goes back to colorless. Examples include ethyne or octyne. Methylene chloride is primarily used as a solvent in paint removers but is also used in aerosol formulations as a solvent in the manufacture of pharmaceuticals as a degreasing agent in. Methylene Chloride is a clear colorless nonflammable volatile liquid chlorinated hydrocarbon with a sweet pleasant smell and emits highly toxic fumes of phosgene when heated to decomposition. Text is available under.
For octyne one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon.
Methylene compound Methyl group. In acetylene the H. Methylene compound Methyl group. This is because owing to the structure the carbon is especially acidic and can easily be deprotonated to form a methylene group. In parent chains with four or more carbons it is necessary to say where the triple bond is located. For octyne one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon.

In parent chains with four or more carbons it is necessary to say where the triple bond is located. Methylene compound Methyl group. The central carbon in 13-dicarbonyl compound is known as an activated methylene group. In acetylene the H. When you shake the solution in a half-filled bottle oxygen goes into the solution oxidizing the methylene blue and turning the solution blue.
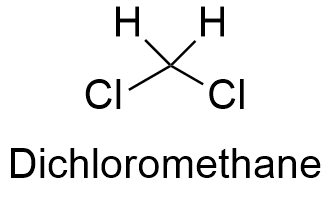 Source: softschools.com
Source: softschools.com
This page was last edited on 3 March 2021 at 2305 UTC. This is a reversible redox reaction. When the shaking stops the oxygen comes out of the solution and it goes back to colorless. This is because owing to the structure the carbon is especially acidic and can easily be deprotonated to form a methylene group. The central carbon in 13-dicarbonyl compound is known as an activated methylene group.

In systematic chemical nomenclature alkynes are named with the Greek prefix system without any additional letters. This page was last edited on 3 March 2021 at 2305 UTC. In parent chains with four or more carbons it is necessary to say where the triple bond is located. For octyne one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon. Methylene chloride is primarily used as a solvent in paint removers but is also used in aerosol formulations as a solvent in the manufacture of pharmaceuticals as a degreasing agent in.

In parent chains with four or more carbons it is necessary to say where the triple bond is located. This is a reversible redox reaction. When the shaking stops the oxygen comes out of the solution and it goes back to colorless. In systematic chemical nomenclature alkynes are named with the Greek prefix system without any additional letters. The blue bottle demonstration involves a solution of glucose sodium hydroxide methylene blue and distilled water.
 Source: oehha.ca.gov
Source: oehha.ca.gov
Text is available under. When you shake the solution in a half-filled bottle oxygen goes into the solution oxidizing the methylene blue and turning the solution blue. In acetylene the H. For octyne one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon. Text is available under.
 Source: fishersci.no
Source: fishersci.no
In parent chains with four or more carbons it is necessary to say where the triple bond is located. In parent chains with four or more carbons it is necessary to say where the triple bond is located. This page was last edited on 3 March 2021 at 2305 UTC. Methylene compound Methyl group. When you shake the solution in a half-filled bottle oxygen goes into the solution oxidizing the methylene blue and turning the solution blue.
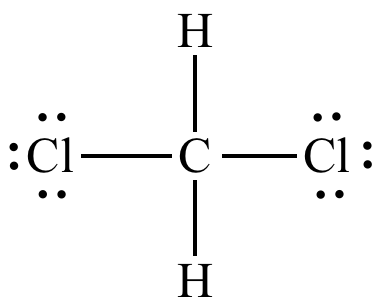 Source: chem.ucla.edu
Source: chem.ucla.edu
For octyne one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon. For octyne one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon. When the shaking stops the oxygen comes out of the solution and it goes back to colorless. The blue bottle demonstration involves a solution of glucose sodium hydroxide methylene blue and distilled water. Methylene chloride is primarily used as a solvent in paint removers but is also used in aerosol formulations as a solvent in the manufacture of pharmaceuticals as a degreasing agent in.
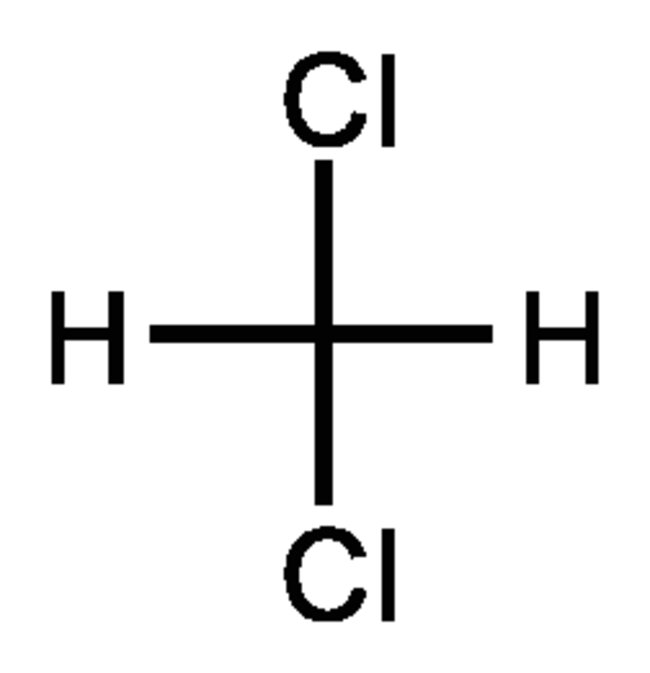 Source: acsh.org
Source: acsh.org
In acetylene the H. Examples include ethyne or octyne. For octyne one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon. When you shake the solution in a half-filled bottle oxygen goes into the solution oxidizing the methylene blue and turning the solution blue. In parent chains with four or more carbons it is necessary to say where the triple bond is located.
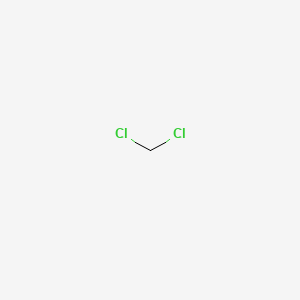
This is because owing to the structure the carbon is especially acidic and can easily be deprotonated to form a methylene group. Methylene compound Methyl group. In systematic chemical nomenclature alkynes are named with the Greek prefix system without any additional letters. In parent chains with four or more carbons it is necessary to say where the triple bond is located. In acetylene the H.
 Source: acs.org
Source: acs.org
The blue bottle demonstration involves a solution of glucose sodium hydroxide methylene blue and distilled water. This is a reversible redox reaction. The central carbon in 13-dicarbonyl compound is known as an activated methylene group. In systematic chemical nomenclature alkynes are named with the Greek prefix system without any additional letters. When you shake the solution in a half-filled bottle oxygen goes into the solution oxidizing the methylene blue and turning the solution blue.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title methylene chloride chemical structure by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.