Molar mass acetic anhydride
Molar Mass Acetic Anhydride. Once the reaction is complete the student collects 247g of aspirin. If molecular formula calculator add up the total value which is 12 46 48 106. 50 mL of 0. On the laboratory scale it can be produced from acetic acid and reagents such as thionyl chloride phosphorus trichloride or phosphorus pentachloride.
 Acetic Anhydride 99 5 108 24 7 From sigmaaldrich.com
Acetic Anhydride 99 5 108 24 7 From sigmaaldrich.com
0 mL of 6. The normal industrial method involves reaction of acetic anhydride with anhydrous hydrogen chloride. The main process involves dehydration of acetic acid to give ketene at 700750 C. Zinc acetates are prepared by the action of acetic acid on zinc carbonate or zinc metal. Zinc acetate is a salt with the formula ZnCH 3 CO 2 2 which commonly occurs as the dihydrate ZnCH 3 CO 2 2 2H 2 O. 10209 gmol Density of acetic anhydride.
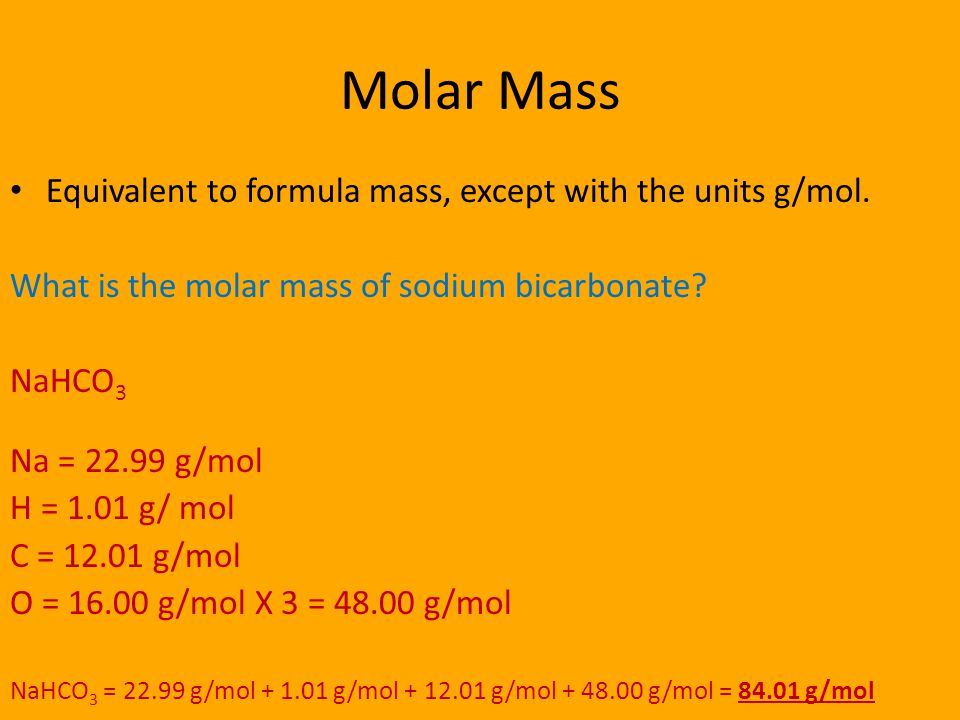
What is the molar mass of sodium carbonate Na2CO3.
In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses. The product of the condensation of two molecules of acetic acid is acetic anhydride. Once the reaction is complete the student collects 247g of aspirin. The normal industrial method involves reaction of acetic anhydride with anhydrous hydrogen chloride. Acetic anhydride ethanol ethyl acetate acetic acid 6 Although sulfuric acid plays a vital role in the esterification reaction mechanism it is beyond the scope of this tutorial. Finally diluted to 500 ml with glacial acetic acid and allowed to stand overnight.
 Source: pdfprof.com
Source: pdfprof.com
120 x 1 12. Zinc acetate is a salt with the formula ZnCH 3 CO 2 2 which commonly occurs as the dihydrate ZnCH 3 CO 2 2 2H 2 O. Therefore the molar mass of Na2CO3 is 106 gmol. Once the reaction is complete the student collects 247g of aspirin. Also important in this field is Avogadros number N.
 Source: chemsynthesis.com
Source: chemsynthesis.com
16 x 3 48. 5 ml of acetic anhydride and allowed the solution to cool for 30 min. The worldwide production of acetic anhydride is a major application and uses approximately 25 to 30 of the global production of acetic acid. Finally diluted to 500 ml with glacial acetic acid and allowed to stand overnight. Sulfuric acid 1 2.

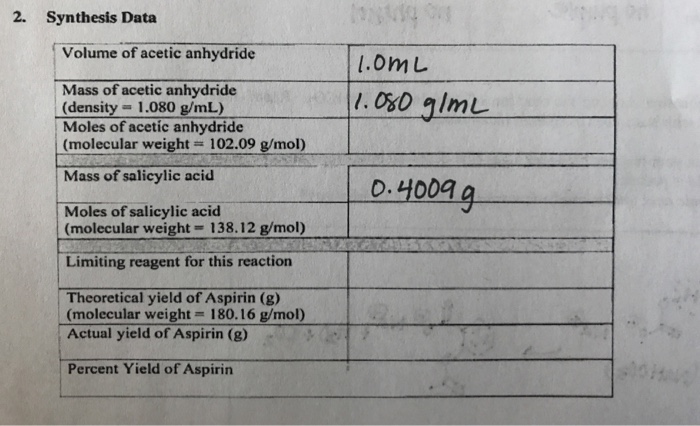
50 mL of 0. In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses. In the NaOH. C4H6O3C7H6O3C9H8O4C2H4O2In a laboratory synthesis a student begins with 500 mL of acetic anhydride density 108 g mL and 208 g of salicylic acid. 10912 gmol Molar mass acetic anhydride.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Since sodium carbonate contains one carbon atom two sodium atoms and three oxygen atoms the molecular weight is. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu. Also important in this field is Avogadros number N. 10912 gmol Molar mass acetic anhydride. 0 mg of nh4cl molar mass 53.

10209 gmol Density of acetic anhydride. The presence of Zn 2 cations led to a substantial increase in the ferrocene conversion. What is the molar mass of sodium carbonate Na2CO3. Molecular mass or molar mass are used in stoichiometry calculations in chemistry. Zinc acetates are prepared by the action of acetic acid on zinc carbonate or zinc metal.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
230 x 2 46. Suffice it to say that since the sulfuric acid that is used during in the reaction is re-produced at the end of the. Zinc acetate is a salt with the formula ZnCH 3 CO 2 2 which commonly occurs as the dihydrate ZnCH 3 CO 2 2 2H 2 O. The product of the condensation of two molecules of acetic acid is acetic anhydride. 230 x 2 46.
In the NaOH. Maleic Anhydride C4H6 13-Butadiene C4H6O3 Acetic Anhydride C4H8 2-Methylpropene C4H8O Tetrahydrofuran C4H8O2 Ethyl Acetate C4H9OH Butyl Alcohol C5H10 Cyclopentane C5H10O5 Ribose C5H12 Pentane C5H12O Methyl Tert-butyl Ether C6H10OS2 Allicin C6H12 Cyclohexane C6H12O Cis-3-Hexen-1-ol C6H12O6 Galactose C6H14 Hexane C6H4Cl2 P-Dichlorobenzene C6H5Br. If molecular formula calculator add up the total value which is 12 46 48 106. 10912 gmol Molar mass acetic anhydride. 16 x 3 48.
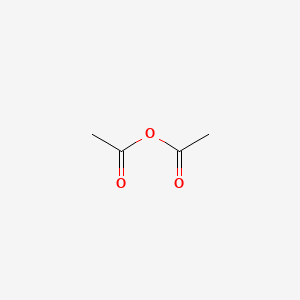
50 mL of 0. I know that the Limiting reactant is C7H6O3. Zinc acetate is a salt with the formula ZnCH 3 CO 2 2 which commonly occurs as the dihydrate ZnCH 3 CO 2 2 2H 2 O. 230 x 2 46. 108 gmL Create an account to start this course today.
 Source: en.wikipedia.org
Source: en.wikipedia.org
0 mL of 6. If molecular formula calculator add up the total value which is 12 46 48 106. On the laboratory scale it can be produced from acetic acid and reagents such as thionyl chloride phosphorus trichloride or phosphorus pentachloride. 50 mL of 0. Molar Mass 310.
 Source: en.wikipedia.org
Source: en.wikipedia.org
10912 gmol Molar mass acetic anhydride. Sulfuric acid 1 2. When used as a food additive it has the E number E650. The worldwide production of acetic anhydride is a major application and uses approximately 25 to 30 of the global production of acetic acid. Molar Mass 310.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass acetic anhydride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.