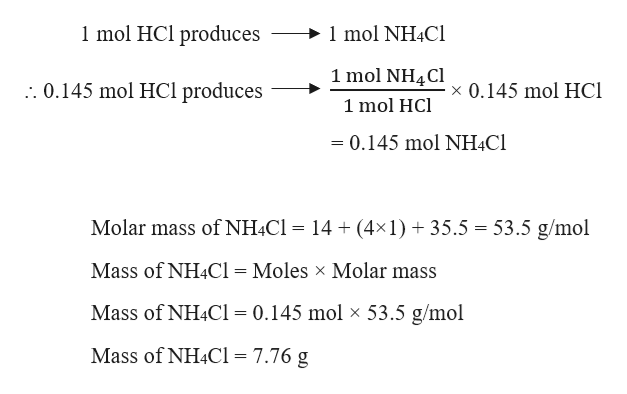
Molar mass ammonium chloride
Molar Mass Ammonium Chloride. A 200 g sample of limestone was dissolved in hydrochloric acid and all the calcium present in the sample was converted to Ca 2 aq. The precipitate was filtered dried and weighed to a constant mass of 243 g. Ammonium Chloride NH 4 Cl- Ammonium chloride is an inorganic compound with formula NH 4 Cl. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound.
 Solution What Mass Of Ammonium Chloride Clutch Prep From clutchprep.com
Solution What Mass Of Ammonium Chloride Clutch Prep From clutchprep.com
Ammonium Chloride NH 4 Cl- Ammonium chloride is an inorganic compound with formula NH 4 Cl. Make sure to show clearly defined and complete work here 1. Conversion between moles and mass. Our molar mass calculator has this for a variety of other compounds. The precipitate was filtered dried and weighed to a constant mass of 243 g. Grams 58443 5 292215 g.
Conversion between moles and mass.
How many moles are in 1225 g of magnesium hydroxide. To learn more about the Structure Properties Preparation Uses and FAQs of Ammonium Chloride NH4Cl Visit BYJUS for more. The precipitate was filtered dried and weighed to a constant mass of 243 g. How many moles are in 245 g of hydrogen gas H2. Ammonium Chloride is a systemic and urinary acidifying salt. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl.
 Source: toppr.com
Source: toppr.com
This acid forming salt also exerts an expectorant effect by irritating the mucous membranes and is used for alleviation of cough. The precipitate was filtered dried and weighed to a constant mass of 243 g. Molar Mass of ammonium chloride NH4Cl Molar Mass of baking powder NaHCO3 Molar Mass of Sucrose C12H22O11 Molar Mass of Isopropyl Alcohol C3H6CHOH Molar Mass of Carbon Monoxide CO Molar Mass of antimony chloride - 3 SbCl3 Molar Mass of Chloroplatinic acid H2PtCl6 Molar Mass of Lactic Acid C3H6O3 Molar Mass of Mercury Sulfate HgSO4 Molar Mass of Potassium. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. This acid forming salt also exerts an expectorant effect by irritating the mucous membranes and is used for alleviation of cough.
 Source: bartleby.com
Source: bartleby.com
Make sure to show clearly defined and complete work here 1. How many moles are in 1225 g of magnesium hydroxide. The action of zinc chlorideammonium chloride fluxes for example in the hot-dip galvanizing process produces H 2 gas and ammonia fumes. To learn more about the Structure Properties Preparation Uses and FAQs of Ammonium Chloride NH4Cl Visit BYJUS for more. Ammonium chloride helps maintain pH and exerts a mild diuretic effect.
 Source: youtube.com
Source: youtube.com
What is the molar mass of. How many grams are in 63 moles of ammonium chloride. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. Our molar mass calculator has this for a variety of other compounds. Molar Mass of Ammonium aluminium sulfate AlNH4SO42 Molar Mass of Ammonium nitrate NH4NO3 Molar Mass of Barium sulfate BaSO4 Molar Mass of Calcium carbonate CaCO3 Molar Mass of carbolic acid C6H5OH Molar Mass of graphite C Molar Mass of methane CH4 Molar Mass of hydrogen cyanide.
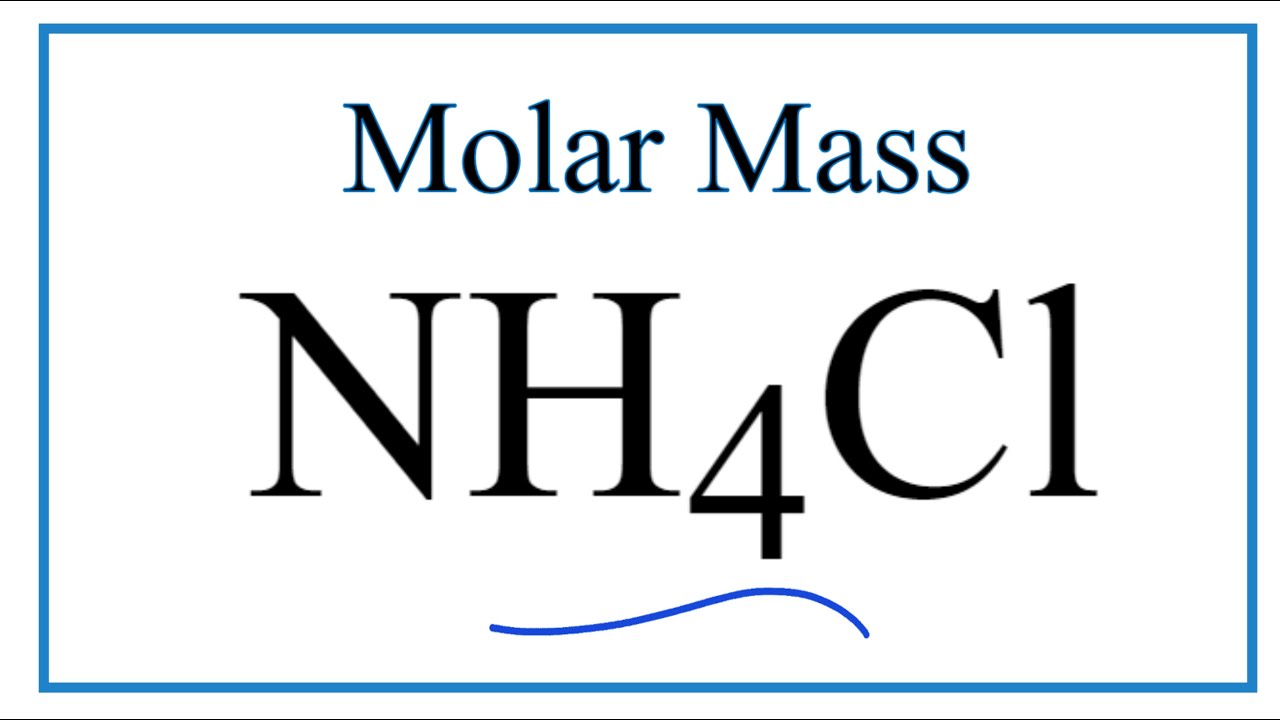 Source: youtube.com
Source: youtube.com
Molar Mass of ammonium chloride NH4Cl Molar Mass of baking powder NaHCO3 Molar Mass of Sucrose C12H22O11 Molar Mass of Isopropyl Alcohol C3H6CHOH Molar Mass of Carbon Monoxide CO Molar Mass of antimony chloride - 3 SbCl3 Molar Mass of Chloroplatinic acid H2PtCl6 Molar Mass of Lactic Acid C3H6O3 Molar Mass of Mercury Sulfate HgSO4 Molar Mass of Potassium. Make sure to show clearly defined and complete work here 1. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. Molar Mass of ammonium chloride NH4Cl Molar Mass of baking powder NaHCO3 Molar Mass of Sucrose C12H22O11 Molar Mass of Isopropyl Alcohol C3H6CHOH Molar Mass of Carbon Monoxide CO Molar Mass of antimony chloride - 3 SbCl3 Molar Mass of Chloroplatinic acid H2PtCl6 Molar Mass of Lactic Acid C3H6O3 Molar Mass of Mercury Sulfate HgSO4 Molar Mass of Potassium. This acid forming salt also exerts an expectorant effect by irritating the mucous membranes and is used for alleviation of cough.
 Source: youtube.com
Source: youtube.com
To learn more about the Structure Properties Preparation Uses and FAQs of Ammonium Chloride NH4Cl Visit BYJUS for more. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Grams 58443 5 292215 g. How many grams are in 63 moles of ammonium chloride. The precipitate was filtered dried and weighed to a constant mass of 243 g.
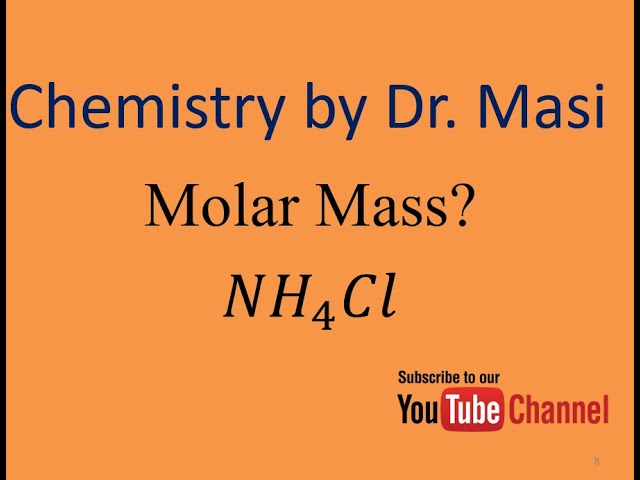 Source: youtube.com
Source: youtube.com
In its pure form it is white crystalline salt. Grams 58443 5 292215 g. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Molar Mass of ammonium chloride NH4Cl Molar Mass of baking powder NaHCO3 Molar Mass of Sucrose C12H22O11 Molar Mass of Isopropyl Alcohol C3H6CHOH Molar Mass of Carbon Monoxide CO Molar Mass of antimony chloride - 3 SbCl3 Molar Mass of Chloroplatinic acid H2PtCl6 Molar Mass of Lactic Acid C3H6O3 Molar Mass of Mercury Sulfate HgSO4 Molar Mass of Potassium. Ammonium Ca2 calcium Fe2 ironII Cu2 copperII Mg2 magnesium Ni2 nickelII Sr2 strontium Zn2 zinc Sn2 tinII Pb2 leadII Ba2 barium Fe3 ironIII Al3 aluminium Cr3 ChromiumIII F-fluoride Cl-chloride Br-bromide I-iodide HO-hydroxide NO 2-nitrite NO 3-nitrate HCO 3 -hydrogencarbonate O2-oxide CO 3 2-carbonate SO 4 2-sulfate HSO 4-hydrogensulfate S2-sulphide N3-nitride PO 4 3.
 Source: clutchprep.com
Source: clutchprep.com
How many grams are in 63 moles of ammonium chloride. Our molar mass calculator has this for a variety of other compounds. Ammonium chloride helps maintain pH and exerts a mild diuretic effect. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. What is the mass of 0288 moles of plumbous.
 Source: slideplayer.com
Source: slideplayer.com
To learn more about the Structure Properties Preparation Uses and FAQs of Ammonium Chloride NH4Cl Visit BYJUS for more. This acid forming salt also exerts an expectorant effect by irritating the mucous membranes and is used for alleviation of cough. Ammonium Chloride is a systemic and urinary acidifying salt. NH 4 2 ZnCl 4 and NH 4 3 ClZnCl 4 which decompose on heating liberating HCl just as zinc chloride hydrate does. Make sure to show clearly defined and complete work here 1.
 Source: slideplayer.com
Source: slideplayer.com
To learn more about the Structure Properties Preparation Uses and FAQs of Ammonium Chloride NH4Cl Visit BYJUS for more. A 200 g sample of limestone was dissolved in hydrochloric acid and all the calcium present in the sample was converted to Ca 2 aq. Zinc chloride forms two salts with ammonium chloride. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. NH 4 2 ZnCl 4 and NH 4 3 ClZnCl 4 which decompose on heating liberating HCl just as zinc chloride hydrate does.
 Source: clutchprep.com
Source: clutchprep.com
Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Ammonium Ca2 calcium Fe2 ironII Cu2 copperII Mg2 magnesium Ni2 nickelII Sr2 strontium Zn2 zinc Sn2 tinII Pb2 leadII Ba2 barium Fe3 ironIII Al3 aluminium Cr3 ChromiumIII F-fluoride Cl-chloride Br-bromide I-iodide HO-hydroxide NO 2-nitrite NO 3-nitrate HCO 3 -hydrogencarbonate O2-oxide CO 3 2-carbonate SO 4 2-sulfate HSO 4-hydrogensulfate S2-sulphide N3-nitride PO 4 3. Ammonium chloride is used in veterinary medicine in the prevention of urinary stones in sheep goats and cattle. What is the mass of 0288 moles of plumbous. In its pure form it is white crystalline salt.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass ammonium chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.