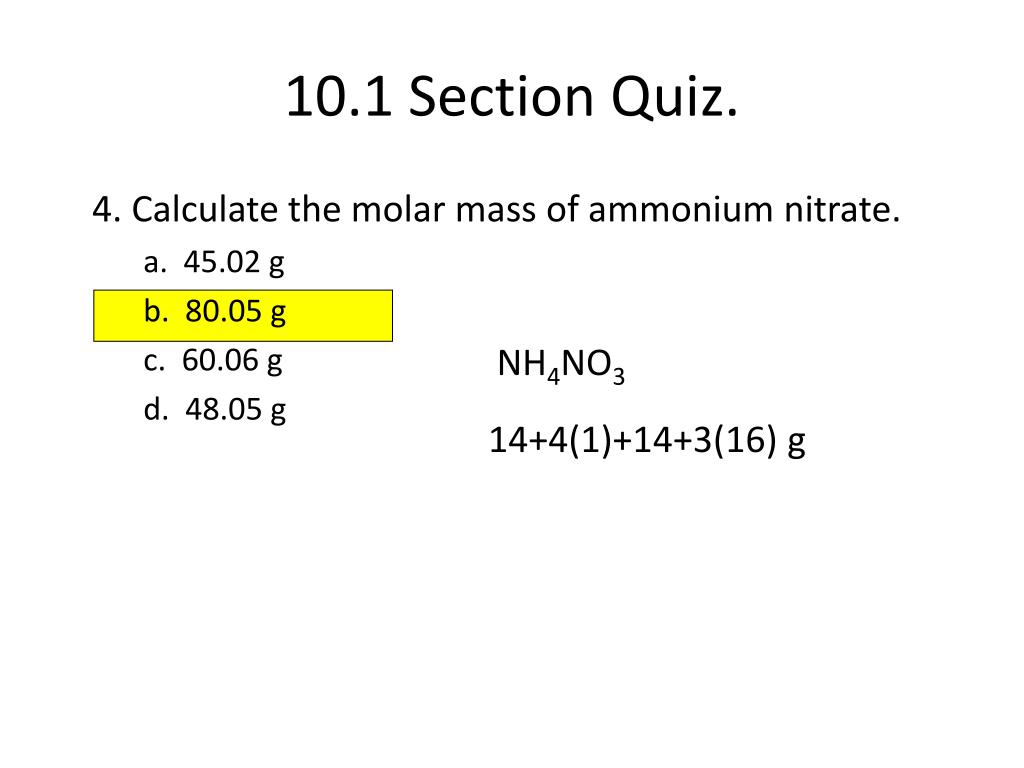
Molar mass ammonium nitrate
Molar Mass Ammonium Nitrate. GHS precautionary statements. 62004 gmol 1 Conjugate acid. Solubility Table Table of Solubilities Water is a commonly used solvent so it is very useful to construct a table of solubilities based on the mass of a solute that will dissolve in a given volume of water. Nitrate is a polyatomic ion with the chemical formula NO 3.

The mass of a mole of any element or compound. Nitrates are common components of fertilizers and explosives. 62004 gmol 1 Conjugate acid. Nitrate is a polyatomic ion with the chemical formula NO 3. 548218 gmol 1 Appearance orange-red crystals Melting point. 107 to 108 C 225 to 226 F.
Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa.
H272 H302 H315 H319 H335. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. Solubility Table Table of Solubilities Water is a commonly used solvent so it is very useful to construct a table of solubilities based on the mass of a solute that will dissolve in a given volume of water. The mass of a mole of any element or compound. H272 H302 H315 H319 H335. 141 g100 mL 25 C 227 g100 mL 80 C Structure Crystal structure.
 Source: youtube.com
Source: youtube.com
Nitrate is a polyatomic ion with the chemical formula NO 3. The mass of a mole of any element or compound. You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute. Salts containing this ion are called nitrates. 141 g100 mL 25 C 227 g100 mL 80 C Structure Crystal structure.
 Source: youtube.com
Source: youtube.com
H272 H302 H315 H319 H335. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. 107 to 108 C 225 to 226 F. 380 to 381 K Solubility in water. 548218 gmol 1 Appearance orange-red crystals Melting point.
 Source: slideserve.com
Source: slideserve.com
Salts containing this ion are called nitrates. 548218 gmol 1 Appearance orange-red crystals Melting point. Nitrates are common components of fertilizers and explosives. 141 g100 mL 25 C 227 g100 mL 80 C Structure Crystal structure. 380 to 381 K Solubility in water.
 Source: youtube.com
Source: youtube.com
62004 gmol 1 Conjugate acid. 548218 gmol 1 Appearance orange-red crystals Melting point. Solubility Table Table of Solubilities Water is a commonly used solvent so it is very useful to construct a table of solubilities based on the mass of a solute that will dissolve in a given volume of water. 62004 gmol 1 Conjugate acid. Salts containing this ion are called nitrates.
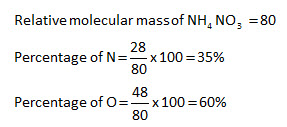 Source: forum.byjus.com
Source: forum.byjus.com
Nitrates are common components of fertilizers and explosives. H272 H302 H315 H319 H335. Nitrates are common components of fertilizers and explosives. 62004 gmol 1 Conjugate acid. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa.

You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute. Nitrates are common components of fertilizers and explosives. GHS precautionary statements. Almost all inorganic nitrates. You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute.
 Source: youtube.com
Source: youtube.com
107 to 108 C 225 to 226 F. Ammonium nitrate 10 M CaOH800 g NH 4NO 3 05 M 400 g 8004 01 M 80 g Ammonium sulfate 01 M 132 g NH 4 2SO 4 1321 Barium chloride 01 M 244 g BaCl 2 2H 2O 24428 Barium hydroxide 01 M 315 3g BaOH 2 8H 2O 31550 Barium nitrate 05 M 1307 g BaNO 3 2 01 M 261 g 26135 Bismuth nitrate 01 M 485 g in BiNO. 380 to 381 K Solubility in water. You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute. H272 H302 H315 H319 H335.
 Source: slideserve.com
Source: slideserve.com
380 to 381 K Solubility in water. H272 H302 H315 H319 H335. Solubility Table Table of Solubilities Water is a commonly used solvent so it is very useful to construct a table of solubilities based on the mass of a solute that will dissolve in a given volume of water. 107 to 108 C 225 to 226 F. 62004 gmol 1 Conjugate acid.
 Source: wiredfaculty.com
Source: wiredfaculty.com
380 to 381 K Solubility in water. The mass of a mole of any element or compound. Nitrates are common components of fertilizers and explosives. 62004 gmol 1 Conjugate acid. 548218 gmol 1 Appearance orange-red crystals Melting point.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute. Solubility Table Table of Solubilities Water is a commonly used solvent so it is very useful to construct a table of solubilities based on the mass of a solute that will dissolve in a given volume of water. You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute. Nitrate is a polyatomic ion with the chemical formula NO 3. 141 g100 mL 25 C 227 g100 mL 80 C Structure Crystal structure.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass ammonium nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.