Molar mass cabr
Molar Mass Cabr. The required change of. That leads to this. What mass of ironll sulfate heptahydrate would completely react with approximately 10 ml of 0010 M KMnO_4. Learn vocabulary terms and more with flashcards games and other study tools.
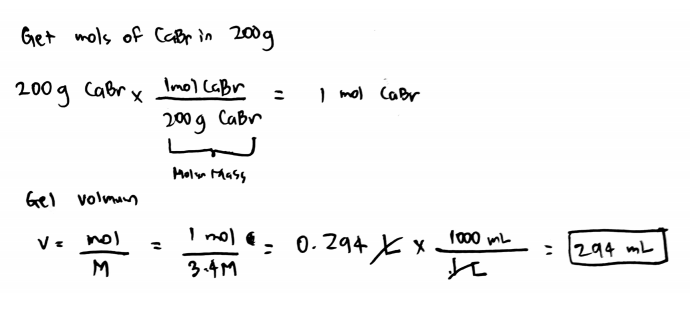 Calculate The Volume In Millimeters Of A 3 Clutch Prep From clutchprep.com
Calculate The Volume In Millimeters Of A 3 Clutch Prep From clutchprep.com
Because this salt is completely dissociated in solution the solution will contain 0022 mol of Ca 2 and 2 0225 067 mol of Br. Now use the number of moles and multiply it by the molar mass. MV mass molar mass x 100 L 200 g 199886 gmol x 0100 M When CaBr 2 ionizes two bromide ions are released for every one CaBr2 that dissolves. The required change of. Return to Solutions Menu. 026 g100ml at 25 C dihydrate Solubility product K sp 493 10 5 mol 2 L 2 anhydrous 314 10 5 dihydrate.
026 g100ml at 25 C dihydrate Solubility product K sp 493 10 5 mol 2 L 2 anhydrous 314 10 5 dihydrate.
Thus the number of moles of CaBr 2 in the solution is 450 g 200 gmol 0225 mol. Weigh out 4473 grams 6 mol of KCl. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. Its action is due to the bromide ion sodium bromide is equally effective. 026 g100ml at 25 C dihydrate Solubility product K sp 493 10 5 mol 2 L 2 anhydrous 314 10 5 dihydrate. Br- 0200 M.
 Source: youtube.com
Source: youtube.com
How many grams are in 0572 moles of glucose C 6 H 12 O 6. Go to Molarity Problems 26-35. Return to Solutions Menu. The Henrys Law constant k for CO2 in water at 25oC is 34 x 10-2 molL atm. The percent composition of the compound is.
Combustion reactions chem worksheet 10 6 answers. Limiting and excess reactants worksheet answers pogil. Academiaedu is a platform for academics to share research papers. Now use the number of moles and multiply it by the molar mass. How many grams are in 379 moles of calcium bromide CaBr 2.
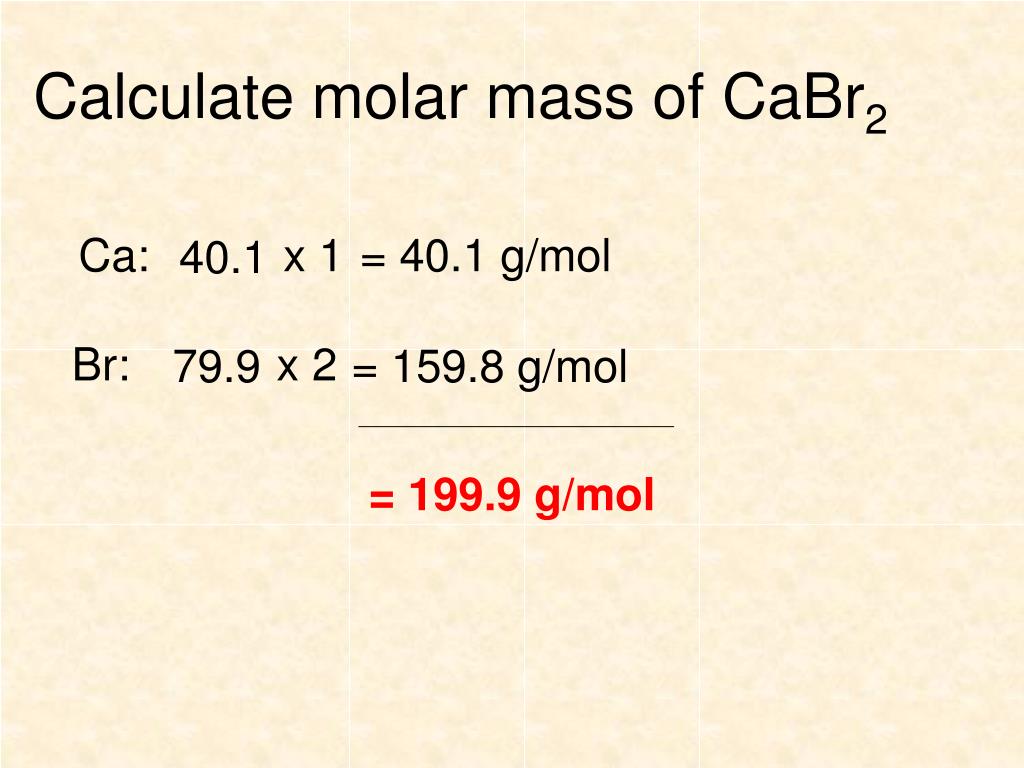 Source: slideserve.com
Source: slideserve.com
What mass of solute exists in a 1L volume of 0650molL-1 CaBr_2. 296 gcm 3 anhydrous 232 gcm 3 dihydrate Melting point. Mass measurements of the sample the isolated analyte or some other component of the analysis system used along with the known stoichiometry of the compounds involved permit calculation of the analyte concentration. Go to Molarity Problems 26-35. If pOH1075 what is the concentration of HO- in the solution.
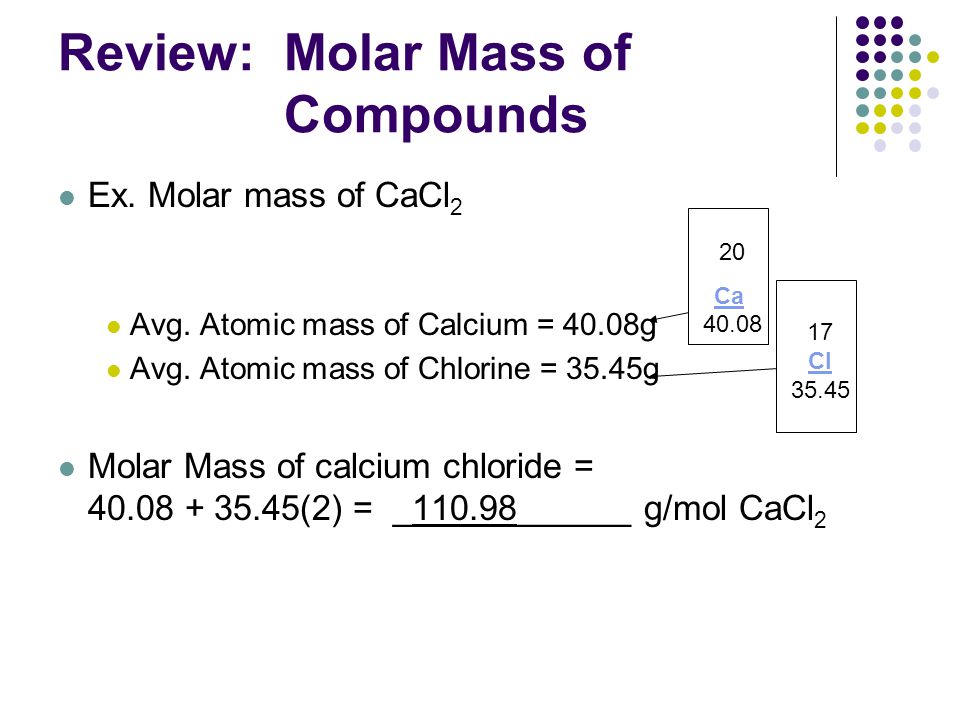 Source: slideplayer.com
Source: slideplayer.com
What mass of ironll sulfate heptahydrate would completely react with approximately 10 ml of 0010 M KMnO_4. Its action is due to the bromide ion sodium bromide is equally effective. H 2g18g x 100 111 O 16g18g x 100 889. Academiaedu is a platform for academics to share research papers. Limiting and excess reactants worksheet answers pogil.
 Source: clutchprep.com
Source: clutchprep.com
Return to Solutions Menu. What mass of ironll sulfate heptahydrate would completely react with approximately 10 ml of 0010 M KMnO_4. Calculate the molar mass of. 13614 gmol anhydrous 14515 gmol hemihydrate 172172 gmol dihydrate Appearance white solid Odor. Potassium bromide K Br is a salt widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries with over-the-counter use extending to 1975 in the US.
 Source: youtube.com
Source: youtube.com
H 2g18g x 100 111 O 16g18g x 100 889. The Henrys Law constant k for CO2 in water at 25oC is 34 x 10-2 molL atm. Determine the molar mass of KCl which is 7455 gmol. 1460 C 2660 F. Because this salt is completely dissociated in solution the solution will contain 0022 mol of Ca 2 and 2 0225 067 mol of Br.
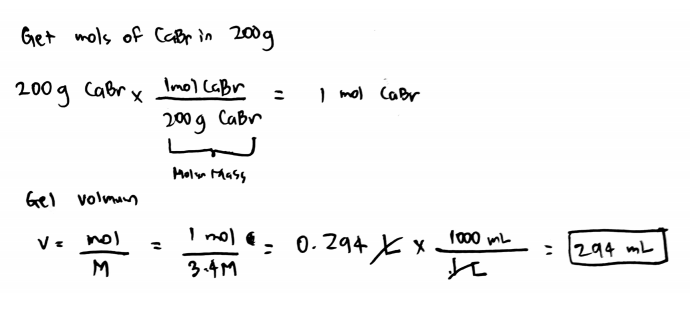 Source: clutchprep.com
Source: clutchprep.com
Learn vocabulary terms and more with flashcards games and other study tools. How many grams are in 0572 moles of glucose C 6 H 12 O 6. Combustion reactions chem worksheet 10 6 answers. What mass of ironll sulfate heptahydrate would completely react with approximately 10 ml of 0010 M KMnO_4. How many grams are in 379 moles of calcium bromide CaBr 2.

If DmassV and molar massmassmoles then molar mass rTDP Molar Mass 08206298K498949 128 gmol 128 x100 4 gmol 100-x100 32 gmol Calculate the concentration of CO2 in a soft drink that is bottled with a partial pressure of CO2 of 40 atm over the liquid solution at 25oC. Combustion reactions chem worksheet 10 6 answers. Weigh out 4473 grams 6 mol of KCl. What mass of solute exists in a 1L volume of 0650molL-1 CaBr_2. The molar mass of H_2O is 18 gmol The hydrogens make up 2g since each mole of hydrogen is 1g The oxygen makes up 16g.
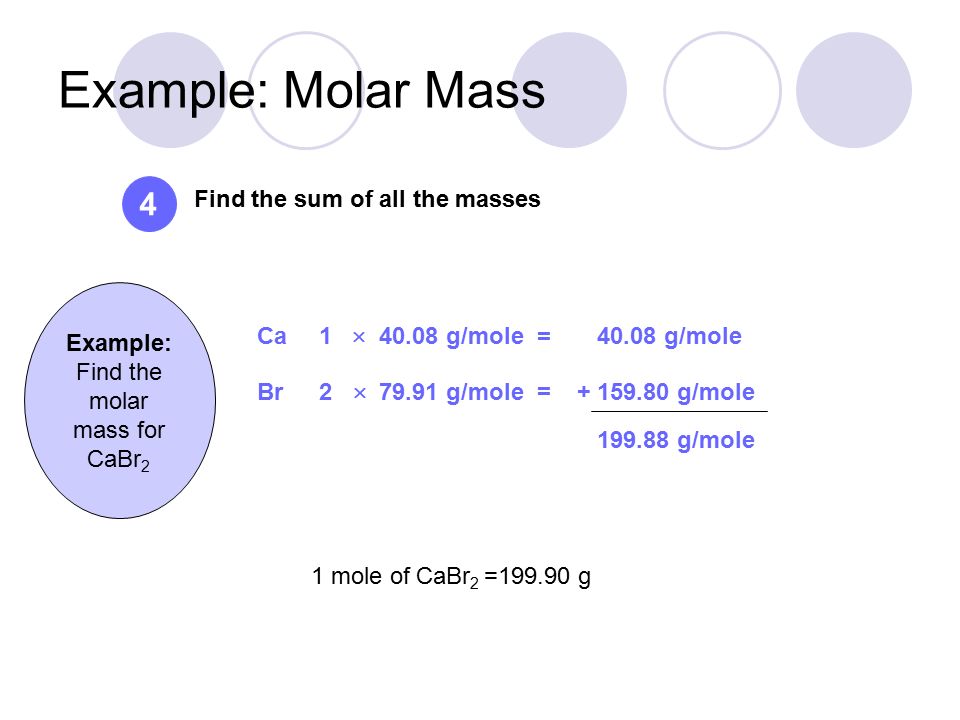 Source: slideplayer.com
Source: slideplayer.com
Chem worksheet 16 1 answers. Potassium bromide is used as a veterinary drug as an antiepileptic medication for dogs. Under standard conditions potassium bromide is a. Thus the number of moles of CaBr 2 in the solution is 450 g 200 gmol 0225 mol. If you need a 15 M solution of calcium bromide eqCaBr_2 eq and have 850 grams of solid eqCaBr_2 eq how many milliliters of solution can you make.
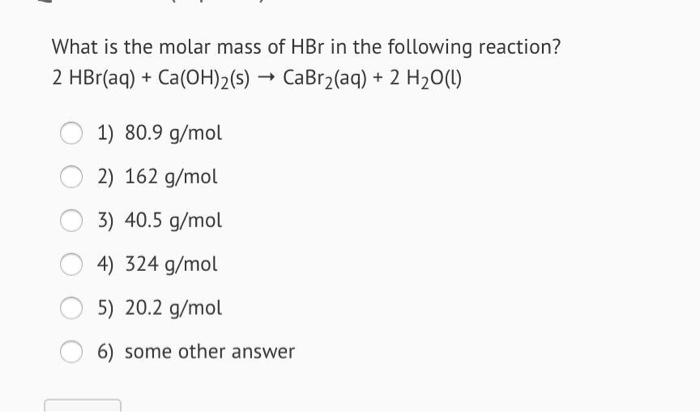 Source: chegg.com
Source: chegg.com
Here is a video which discusses how to calculate percent. 1730 K anhydrous Solubility in water. Because this salt is completely dissociated in solution the solution will contain 0022 mol of Ca 2 and 2 0225 067 mol of Br. What mass of sodium hydroxide NaOH is contained in 250cm3 of 002M sodium hydroxide solution. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass cabr by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






