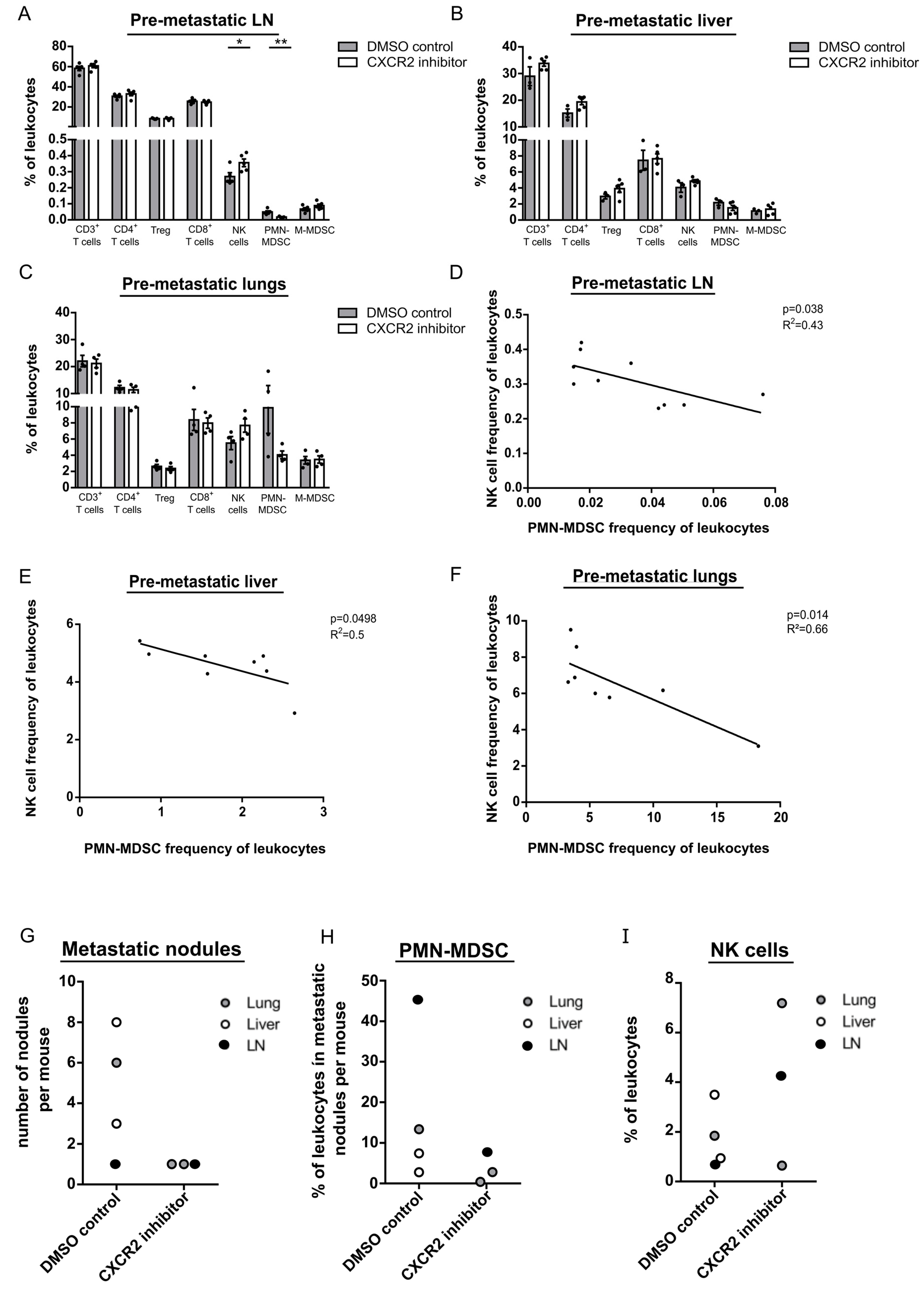
Molar mass copper ii sulfate
Molar Mass Copper Ii Sulfate. For example copperII pentahydrate CuSO_4 5H_2O is blue. TetraamminecopperII sulfate is the salt with the formula CuNH 3 4SO 4 H 2 O. Mol What information has been given in the question. Now the difference between hydrated copperII sulfate and anhydrous copperII sulfate except for the fact that the former contains water of crystallization is the color.
 Answered Calculate The Molar Mass Of Cuso4 Bartleby From bartleby.com
Answered Calculate The Molar Mass Of Cuso4 Bartleby From bartleby.com
CopperII sulfateVI-5-water CuSO 45H 2 Os HARMFUL DANGEROUS FOR THE ENVIRONMENT see CLEAPSS Hazcard HC027c. What is the question asking you to calculate. N moles of solute. This dark blue to purple solid is a salt of the metal complex CuNH 3 4 H 2 O 2It is closely related to Schweizers reagent which is used for the production of cellulose fibers in the production of rayon. In the case of copperII sulfate hydrates you only get x as a whole number. Crystals of copperII chloride in a container It is made by reacting copper with chlorine.
Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and.
TetraamminecopperII sulfate is the salt with the formula CuNH 3 4SO 4 H 2 O. FePO 4 2H 2 O. N moles of solute. Mol What information has been given in the question. In the case of copperII sulfate hydrates you only get x as a whole number. Extract the data from the question.
 Source: youtube.com
Source: youtube.com
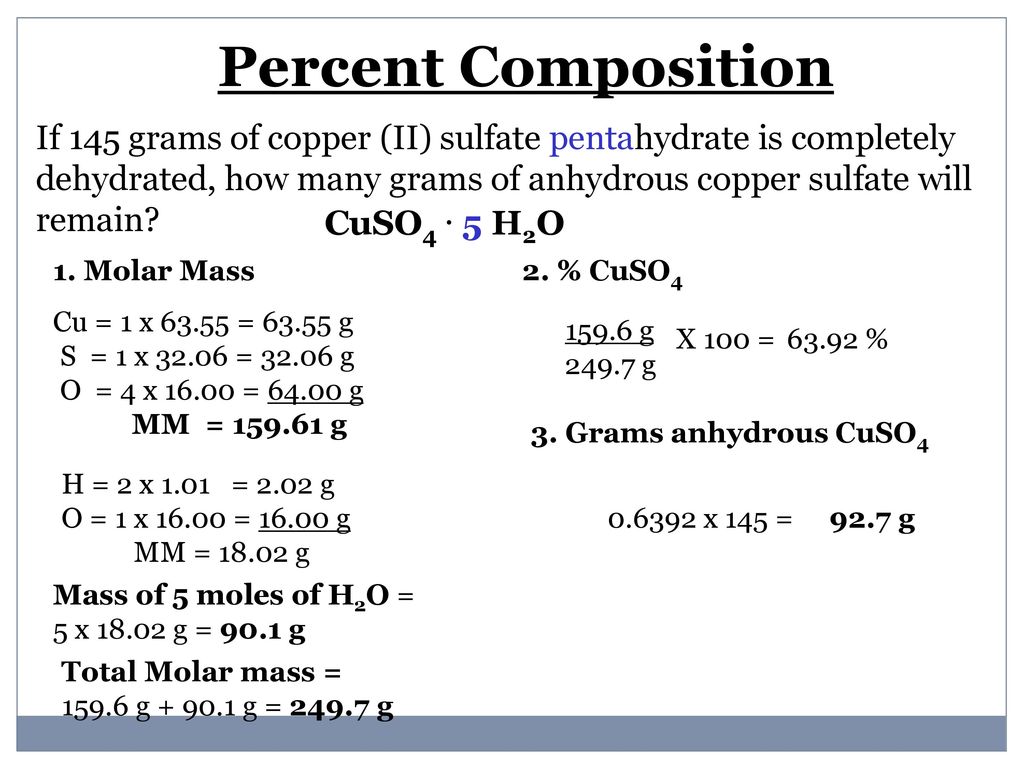
For example copperII pentahydrate CuSO_4 5H_2O is blue. In the case of copperII sulfate hydrates you only get x as a whole number. What is the question asking you to calculate. TetraamminecopperII sulfate is the salt with the formula CuNH 3 4SO 4 H 2 O. On the other hand.
 Source: slideplayer.com
Source: slideplayer.com
N moles of solute. Extract the data from the question. CopperII sulfateVI-5-water CuSO 45H 2 Os HARMFUL DANGEROUS FOR THE ENVIRONMENT see CLEAPSS Hazcard HC027c. TetraamminecopperII sulfate is the salt with the formula CuNH 3 4SO 4 H 2 O. Crystals of copperII chloride in a container It is made by reacting copper with chlorine.
 Source: youtube.com
Source: youtube.com
N moles of solute. In the case of copperII sulfate hydrates you only get x as a whole number. It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction. Crystals of copperII chloride in a container It is made by reacting copper with chlorine. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and.
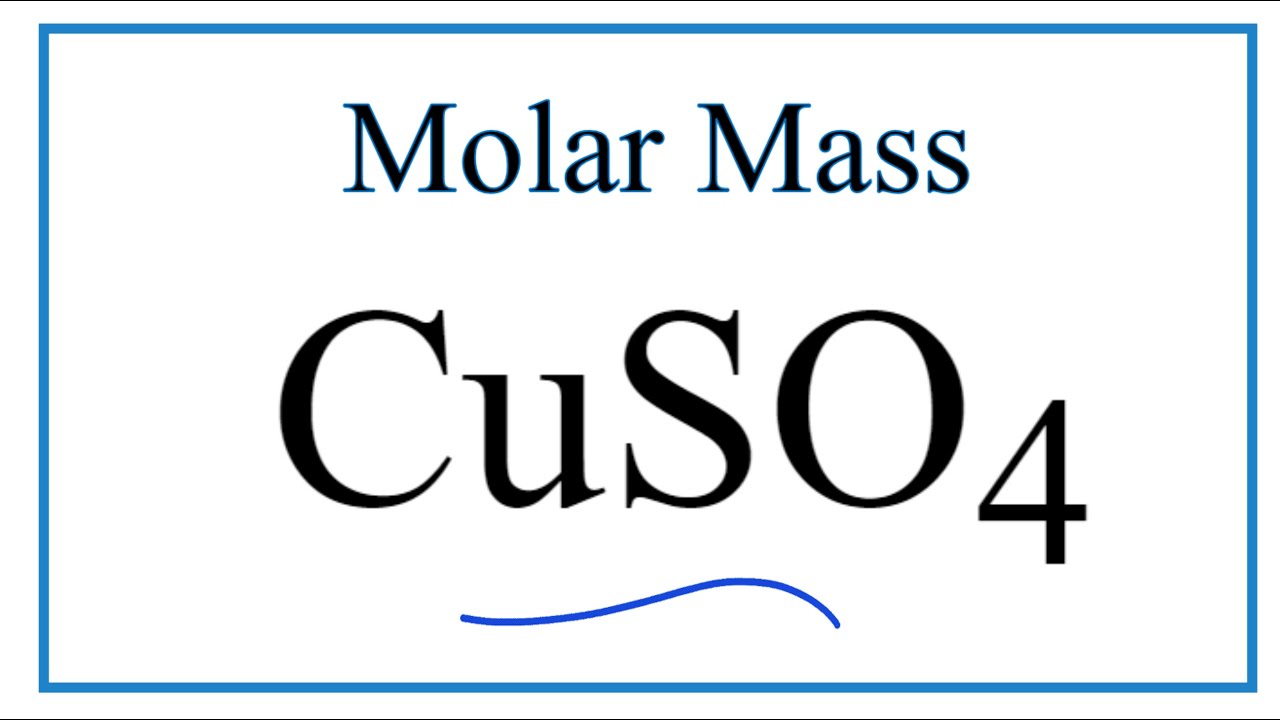 Source: youtube.com
Source: youtube.com
C concentration molarity of solution 0020 mol L-1 V volume of solution 25000 mL 25000 1000 25000 x 10-3 L 025 L since. Now the difference between hydrated copperII sulfate and anhydrous copperII sulfate except for the fact that the former contains water of crystallization is the color. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and. Royal Society of Chemistry. C concentration molarity of solution 0020 mol L-1 V volume of solution 25000 mL 25000 1000 25000 x 10-3 L 025 L since.
 Source: youtube.com
Source: youtube.com
For example copperII pentahydrate CuSO_4 5H_2O is blue. Potassium permanganate is an inorganic compound with the chemical formula KMnO 4 and composed of K and MnO 4It is a purplish-black crystalline salt that dissolves in water to give intensely pink or purple solutions. Now the difference between hydrated copperII sulfate and anhydrous copperII sulfate except for the fact that the former contains water of crystallization is the color. C concentration molarity of solution 0020 mol L-1 V volume of solution 25000 mL 25000 1000 25000 x 10-3 L 025 L since. On the other hand.
 Source: bartleby.com
Source: bartleby.com
Extract the data from the question. Calculate the moles of copper sulfate in 25000 mL of 0020 mol L-1 copper sulfate solution. On the other hand. What is the question asking you to calculate. Set up the apparatus as shown but without water in the receiving tube this is to be collected during the experiment placing about 5 g of powdered hydrated copperII sulfate in the test tube.
 Source: socratic.org
Source: socratic.org
What is the question asking you to calculate. Crystals of copperII chloride in a container It is made by reacting copper with chlorine. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and. TetraamminecopperII sulfate is the salt with the formula CuNH 3 4SO 4 H 2 O. What is the question asking you to calculate.
 Source: slideplayer.com
Source: slideplayer.com
For example copperII pentahydrate CuSO_4 5H_2O is blue. Royal Society of Chemistry. For example copperII pentahydrate CuSO_4 5H_2O is blue. C concentration molarity of solution 0020 mol L-1 V volume of solution 25000 mL 25000 1000 25000 x 10-3 L 025 L since. On the other hand.
 Source: youtube.com
Source: youtube.com
On the other hand. FePO 4 2H 2 O. Mol What information has been given in the question. C concentration molarity of solution 0020 mol L-1 V volume of solution 25000 mL 25000 1000 25000 x 10-3 L 025 L since. What is the question asking you to calculate.
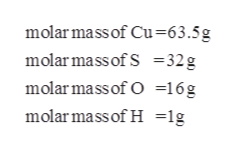 Source: bartleby.com
Source: bartleby.com
FePO 4 2H 2 O. N moles of solute. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and. C concentration molarity of solution 0020 mol L-1 V volume of solution 25000 mL 25000 1000 25000 x 10-3 L 025 L since. Calculate the moles of copper sulfate in 25000 mL of 0020 mol L-1 copper sulfate solution.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass copper ii sulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.