Molar mass copper sulfate
Molar Mass Copper Sulfate. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. Zinc sulfate is formed and is aqueous. Copper Sulfate and water formed. Insoluble in ethanol cold dilute acids.
 Percent Composition Practice Problem 3 Cuso4 5h2o Youtube From youtube.com
Percent Composition Practice Problem 3 Cuso4 5h2o Youtube From youtube.com
747 K Solubility in water. 89563 gmol Appearance off-white pale yellow powder Density. 474 C 885 F. Both solutions contain the same mass of copper nitrate. Anhydrous CuSO 4 has a grey-white powdery appearance whereas the pentahydrate has a bright blue colour. Mark Ott Dilution is also a common means of preparing solutions of a desired concentration.
Both solutions contain the same mass of copper nitrate.
Net Ionic Equations 1. Soluble in NH 4 OH KCN. The law that the scientist used to predict that the product of the reaction would be 159 g of copper sulfate is the law of conservation of matter mass The la Sky0607 Sky0607 10122021 Chemistry High School answered expert verified 50 POINTS. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. Anhydrous CuSO 4 has a grey-white powdery appearance whereas the pentahydrate has a bright blue colour. Copper Cu reacts with sulfur S to form copper sulfide as shown in the equation.
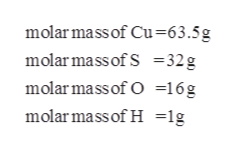 Source: bartleby.com
Source: bartleby.com
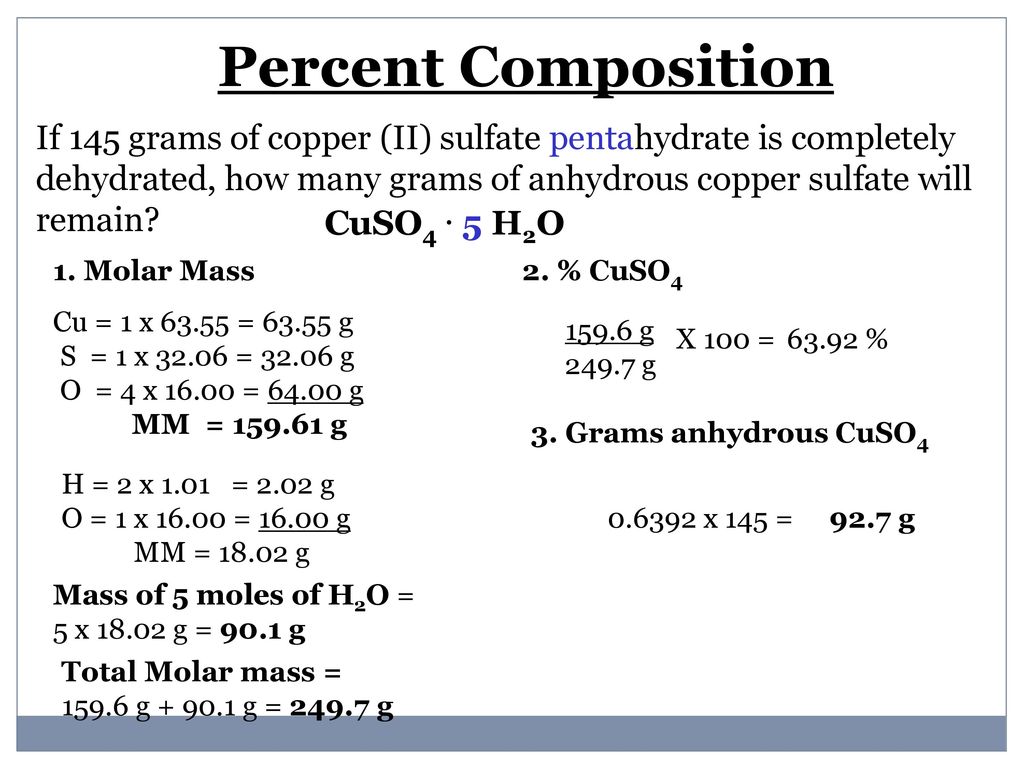
The molar mass of the anhydrous and the pentahydrate forms of copper sulfate are 159609 gramsmole and 249685 grams per mole respectively. By adding solvent to a measured portion of a more concentrated stock solution we can achieve a particular concentration. Mark Ott Dilution is also a common means of preparing solutions of a desired concentration. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. On the other hand.
 Source: slideplayer.com
Source: slideplayer.com
On the other hand. Copper Cu reacts with sulfur S to form copper sulfide as shown in the equation. On the other hand. Cus 4H aq 2NO 3-aq— Cu 2 aq 2NO. The loss in mass of the compound after.
 Source: youtube.com
Source: youtube.com
Molar Mass of Frequently Calculated Chemicals. Net Ionic Equations 1. Cu s HNO 3 aq NIE. The molar mass of the anhydrous and the pentahydrate forms of copper sulfate are 159609 gramsmole and 249685 grams per mole respectively. GHS Signal word.
 Source: slideplayer.com
Source: slideplayer.com
Monoclinic Hazards Safety data sheet. By adding solvent to a measured portion of a more concentrated stock solution we can achieve a particular concentration. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner. Molar Mass of Frequently Calculated Chemicals.
 Source: youtube.com
Source: youtube.com
Negligible Solubility product K sp 347 10 20. The law that the scientist used to predict that the product of the reaction would be 159 g of copper sulfate is the law of conservation of matter mass The la Sky0607 Sky0607 10122021 Chemistry High School answered expert verified 50 POINTS. Both solutions contain the same mass of copper nitrate. For example copperII pentahydrate CuSO_4 5H_2O is blue. Anhydrous CuSO 4 has a grey-white powdery appearance whereas the pentahydrate has a bright blue colour.
 Source: youtube.com
Source: youtube.com
Copper Sulfate and water formed. Net Ionic Equations 1. By adding solvent to a measured portion of a more concentrated stock solution we can achieve a particular concentration. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. For example copperII pentahydrate CuSO_4 5H_2O is blue.
 Source: youtube.com
Source: youtube.com
By adding solvent to a measured portion of a more concentrated stock solution we can achieve a particular concentration. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. Copper Cu reacts with sulfur S to form copper sulfide as shown in the equation. Mark Ott Dilution is also a common means of preparing solutions of a desired concentration. By adding solvent to a measured portion of a more concentrated stock solution we can achieve a particular concentration.
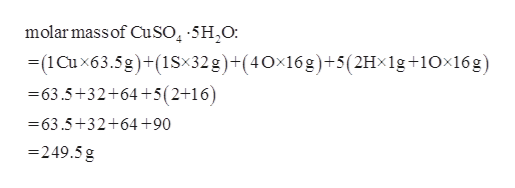 Source: bartleby.com
Source: bartleby.com
Cu s HNO 3 aq NIE. By adding solvent to a measured portion of a more concentrated stock solution we can achieve a particular concentration. The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner. Cu s HNO 3 aq NIE. Insoluble in ethanol cold dilute acids.
 Source: youtube.com
Source: youtube.com
The molar mass of the anhydrous and the pentahydrate forms of copper sulfate are 159609 gramsmole and 249685 grams per mole respectively. Hydrogen gas is also formed and released. Net Ionic Equations 1. On the other hand. Copper Sulfate and water formed.
 Source: tutorpace.com
Source: tutorpace.com
Copper Cu reacts with sulfur S to form copper sulfide as shown in the equation. For instance the copper sulfate used earlier in the semester was stated to be CuSO 4 but it actually had absorbed 5 moles of water for every 1 mole of CuSO 4 and should have been correctly labeled as CuSO 45H 2O copper II sulfate pentahydrate. Zinc sulfate is formed and is aqueous. By adding solvent to a measured portion of a more concentrated stock solution we can achieve a particular concentration. The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass copper sulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






