Molar mass of 1 propanol
Molar Mass Of 1 Propanol. Tert-Butyl alcohol is the simplest tertiary alcohol with a formula of CH 3 3 COH sometimes represented as t-BuOHIt is one of the four isomers of butanol. Temperature K A B C Reference Comment. Flash point 85 - 100F. From the table we see that 1 mole of methane gas CH 4g undergoes complete combustion in excess oxygen gas releasing 890 kJ of heat.
 Molecular Interactions In Binary Mixtures Of 1 Butoxy 2 Propanol With Alcohols At Different Temperatures A Thermophysical And Spectroscopic Approach Sciencedirect From sciencedirect.com
Molecular Interactions In Binary Mixtures Of 1 Butoxy 2 Propanol With Alcohols At Different Temperatures A Thermophysical And Spectroscopic Approach Sciencedirect From sciencedirect.com
Table 91 Comparison of Molar Mass and Boiling Points. Tert-Butyl alcohol is a colorless solid which melts near room temperature and has a camphor-like odorIt is miscible with water ethanol and diethyl ether. Jump to main content Jump to site nav. Less dense than water. Intermolecular forces in 1 propanol. Compare the solubilities of carboxylic acids in water with the solubilities of comparable alkanes and alcohols in water.
The heat of combustion of ethanol ΔH c C 2 H 6 O l 136691kJmol 1000gkg 4808 gmol 29664 kJkg ethanol 297 MJkg 12754 BTUlb 7086 kcalkg.
Isobutanol is an alkyl alcohol that is propan-1-ol substituted by a methyl. The carboxylic acids with 5 to 10 carbon atoms all have goaty odors explaining the odor of Limburger cheese. The molweight of ethanol is 21201 6101 11600 4608 gmol. Jump to main content Jump to site nav. However gas molecules are not point masses and there are many cases gases need to be treated as non-idealJohannes D. From the table we see that 1 mole of methane gas CH 4g undergoes complete combustion in excess oxygen gas releasing 890 kJ of heat.
 Source: en.wikipedia.org
Source: en.wikipedia.org
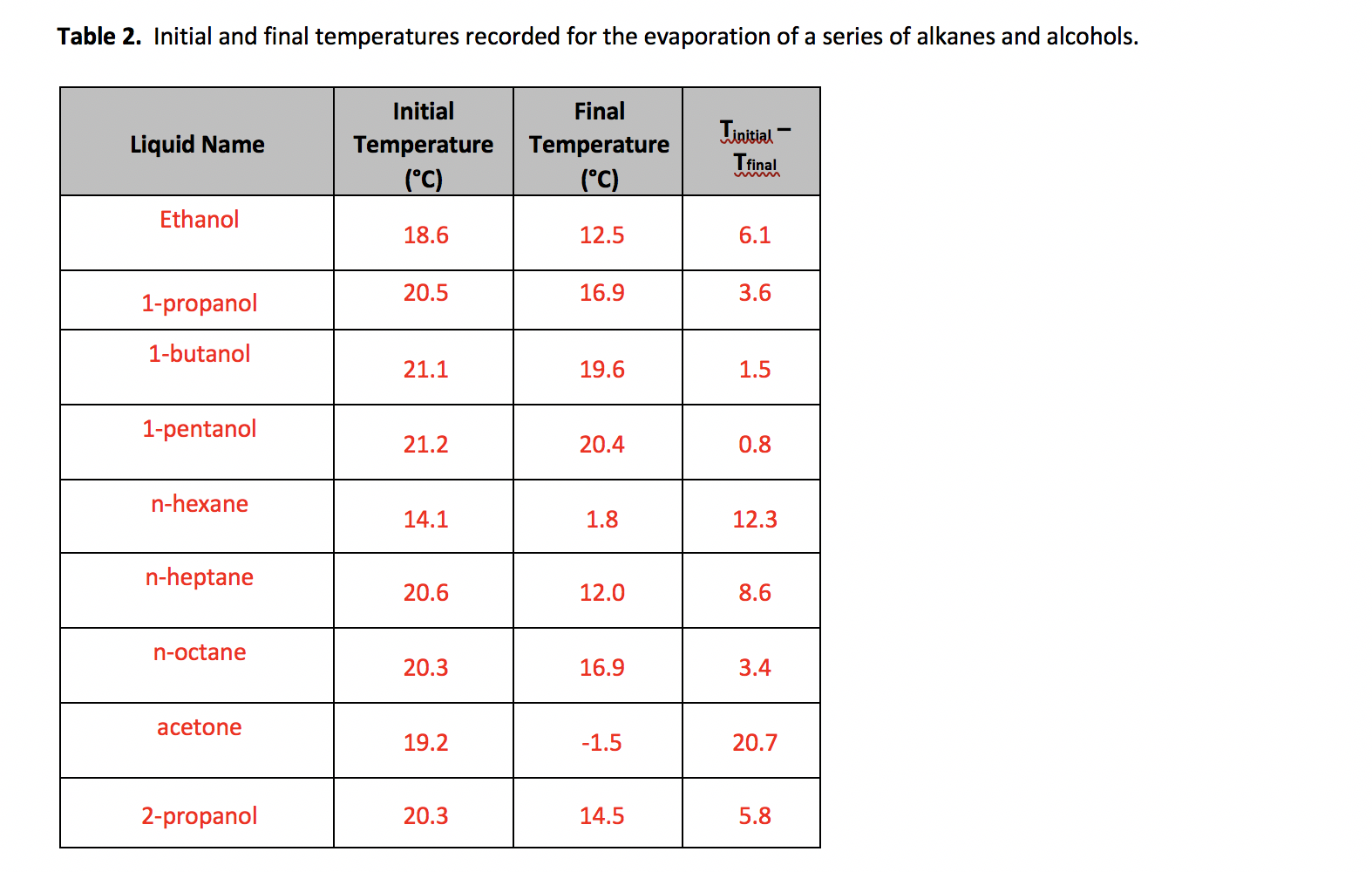
Alkanes are nonpolar and are thus associated only through relatively weak London Dispersion Forces LDFs. Alkanes are nonpolar and are thus associated only through relatively weak London Dispersion Forces LDFs. 1-Propanol Propan-1-ol 71-23-8 71-31-8 109-78-4 927-74-2 36294-23-2. Temperature K A B C Reference Comment. Urine was analyzed immediately 1 2 8 and 9 hr after drinking during 2 hr 375 mlkg of beverages containing orange juice 15 or 40 ethanol and and 1 gl of 1-propanol 2-propanol 1-butanol 2-butanol isobutyl alcohol or a mixture of 1-propanol isobutyl alcohol.
 Source: alchetron.com
Source: alchetron.com
Isobutanol appears as a clear colorless liquid with a sweet odor. Isobutanol is an alkyl alcohol that is propan-1-ol substituted by a methyl. Membership. 1-Propanol is a primary alcohol with the formula CH 3 CH 2 CH 2 OH and sometimes represented as PrOH or n-PrOHIt is a colorless liquid and an isomer of 2-propanolIt is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry mainly for resins and cellulose esters and sometimes as a disinfecting agent. Isobutanol appears as a clear colorless liquid with a sweet odor.
 Source: sciencedirect.com
Source: sciencedirect.com
Flash point 85 - 100F. Urine was analyzed immediately 1 2 8 and 9 hr after drinking during 2 hr 375 mlkg of beverages containing orange juice 15 or 40 ethanol and and 1 gl of 1-propanol 2-propanol 1-butanol 2-butanol isobutyl alcohol or a mixture of 1-propanol isobutyl alcohol. 1-propanol CH 3 OCH 2 CH 3 methyl ethyl ether amide O O C OH O C O O C NH N O C O CH 3 CH 2 C H propanal O R R C NH R R N R O R C OH ketone organic acid ester amine O CH 3 CH 2 C OH propanoic acid O CH 3 CH 2 COCH 3 methyl propanoate CH 3 CH 2 CH 2 NH 2 1-propanamine O CH 3 CH 2 C NH propanamide Note. Flash point 85 - 100F. C 3 H 7 OH l 9 2 O 2g 3CO 2g 4H 2 O l ΔH -2021.

Flash point 85 - 100F. Jump to main content Jump to site nav. A particle-level diagram of a metallic element is shown above. Ambrose Sprake et al 1975. R represents a bonded atom or.
 Source: chegg.com
Source: chegg.com
Temperature K A B C Reference Comment. The molar heat of combustion of methane gas is given in the table as a positive. Journals books. From the table we see that 1 mole of methane gas CH 4g undergoes complete combustion in excess oxygen gas releasing 890 kJ of heat. Alkanes are nonpolar and are thus associated only through relatively weak London Dispersion Forces LDFs.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The table above shows the structural formulas and molar masses for three different compounds. Less dense than water. Butane 1-propanol acetone B. Which of the following is a list of the compounds in order of increasing boiling points. Butane acetone 1-propanol C.

Many carboxylic acids are colorless liquids with disagreeable odors. The molweight of ethanol is 21201 6101 11600 4608 gmol. R represents a bonded atom or. Isobutanol is an alkyl alcohol that is propan-1-ol substituted by a methyl. 1-Propanol Propan-1-ol 71-23-8 71-31-8 109-78-4 927-74-2 36294-23-2.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Coefficents calculated by NIST from authors data. Isobutanol appears as a clear colorless liquid with a sweet odor. The carboxylic acids with 5 to 10 carbon atoms all have goaty odors explaining the odor of Limburger cheese. In contrast if we analyze the compounds that contain an alcohol functional group even methanol with. Structure properties spectra suppliers and links for.

1-propanol acetone butane DAcetone butane 1-propanol. Table 91 Comparison of Molar Mass and Boiling Points. The molar heat of combustion of methane gas is given in the table as a positive. From the table we see that 1 mole of methane gas CH 4g undergoes complete combustion in excess oxygen gas releasing 890 kJ of heat. Butane 1-propanol acetone B.
 Source: chegg.com
Source: chegg.com
Table 91 Comparison of Molar Mass and Boiling Points. The table below shows values of heat of combustion calculated after the above described method. The boiling points of alkanes with one to four carbon atoms are so low that all of these molecules are gases at room temperature. Tert-Butyl alcohol is a colorless solid which melts near room temperature and has a camphor-like odorIt is miscible with water ethanol and diethyl ether. Jump to main content Jump to site nav.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass of 1 propanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.