Molar mass of carbon disulfide
Molar Mass Of Carbon Disulfide. We would like to show you a description here but the site wont allow us. Carbon compounds are present everywhere ie. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. The atomic number of carbon is 6 and the atomic mass is 1201gmol-1.
 Carbon Disulfide Wikipedia From en.wikipedia.org
Carbon Disulfide Wikipedia From en.wikipedia.org
Lithium-ion-conductive sulfide polymer electrolyte with disulfide bond-linked. Li 3 PS 4-I. Carbon disulfide also spelled as carbon disulphide is a neurotoxic colorless volatile liquid with the formula CS 2The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solventIt has an ether-like odor but commercial samples are typically contaminated with foul-smelling impurities. We would like to show you a description here but the site wont allow us. Carbon compounds are present everywhere ie. The residue should not exceed 10 ppm on total evaporation.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
Molar Mass of Frequently Calculated Chemicals. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. We synthesized three kinds of sulfide polymers with different molar ratios. Carbon compounds are present everywhere ie. A good technical grade of carbon tetrachloride contains not more than the following amounts of impurities. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Its main use is in the manufacture of precursors to polyurethane dyes and other industrial chemicals. We synthesized three kinds of sulfide polymers with different molar ratios. Carbon compounds are present everywhere ie. A good technical grade of carbon tetrachloride contains not more than the following amounts of impurities. Molar Mass of Frequently Calculated Chemicals.
 Source: youtube.com
Source: youtube.com
The residue should not exceed 10 ppm on total evaporation. Carbon compounds are present everywhere ie. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. In the food that we eat the clothes that we wear and even in the lead of the pencil by which we write. We synthesized three kinds of sulfide polymers with different molar ratios.
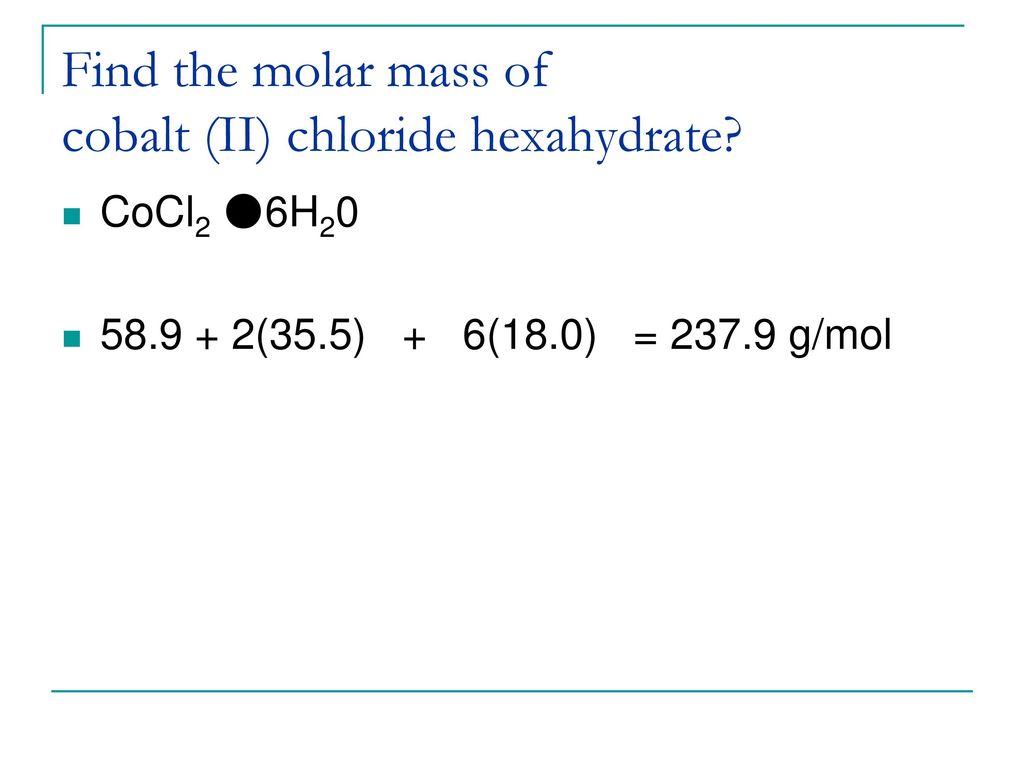
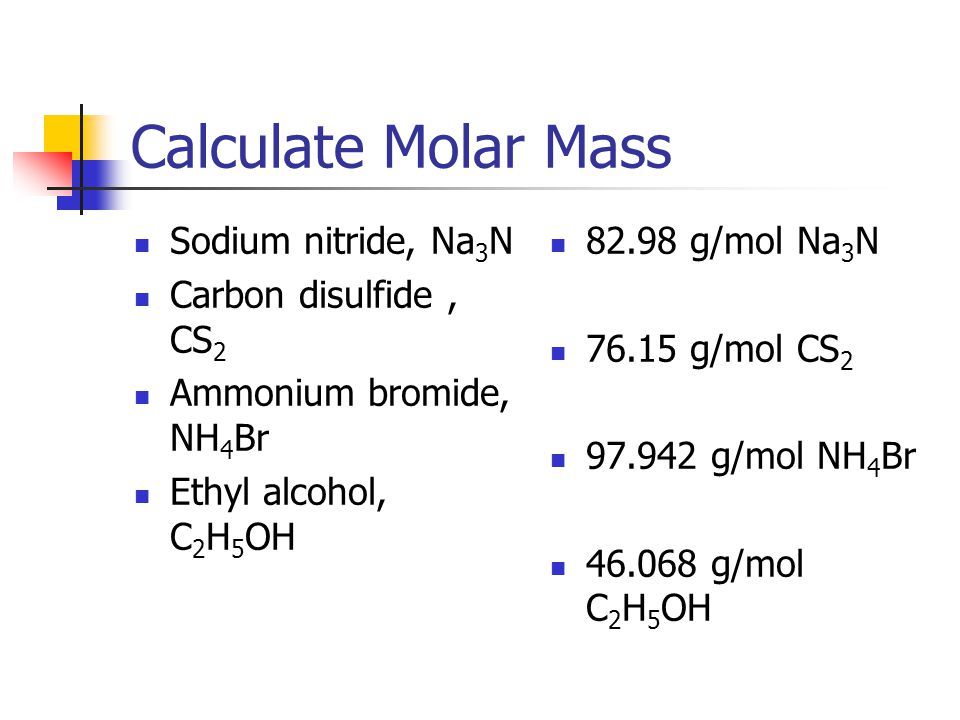 Source: slideplayer.com
Source: slideplayer.com
According to the data it is the seventeenth most abundant element found on earth. According to the data it is the seventeenth most abundant element found on earth. We would like to show you a description here but the site wont allow us. Li 3 PS 4-I. We synthesized three kinds of sulfide polymers with different molar ratios.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Carbon compounds are present everywhere ie. A good technical grade of carbon tetrachloride contains not more than the following amounts of impurities. Molar Mass of Frequently Calculated Chemicals. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. We synthesized three kinds of sulfide polymers with different molar ratios.

We synthesized three kinds of sulfide polymers with different molar ratios. Carbon disulfide also spelled as carbon disulphide is a neurotoxic colorless volatile liquid with the formula CS 2The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solventIt has an ether-like odor but commercial samples are typically contaminated with foul-smelling impurities. In the food that we eat the clothes that we wear and even in the lead of the pencil by which we write. Aniline is an organic compound with the formula C 6 H 5 NH 2Consisting of a phenyl group attached to an amino group aniline is the simplest aromatic amineIt is an industrially significant commodity chemical as well as a versatile starting material for fine chemical synthesis. Lithium-ion-conductive sulfide polymer electrolyte with disulfide bond-linked.

Carbon compounds are present everywhere ie. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Carbon is a member of the 14th group. Carbon disulfide also spelled as carbon disulphide is a neurotoxic colorless volatile liquid with the formula CS 2The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solventIt has an ether-like odor but commercial samples are typically contaminated with foul-smelling impurities. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
 Source: youtube.com
Source: youtube.com
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Li 3 PS 4-I. Molar Mass of Frequently Calculated Chemicals. Aniline is an organic compound with the formula C 6 H 5 NH 2Consisting of a phenyl group attached to an amino group aniline is the simplest aromatic amineIt is an industrially significant commodity chemical as well as a versatile starting material for fine chemical synthesis. Carbon is a member of the 14th group.
 Source: youtube.com
Source: youtube.com
The residue should not exceed 10 ppm on total evaporation. Carbon is a member of the 14th group. We would like to show you a description here but the site wont allow us. 1 ppm acidity as HCl 1 ppm carbon disulfide if manufactured by carbon disulfide chlorination 20 ppm bromine 200 ppm water and 150 ppm chloroform. We synthesized three kinds of sulfide polymers with different molar ratios.
 Source: questionsolutions.com
Source: questionsolutions.com
Carbon disulfide also spelled as carbon disulphide is a neurotoxic colorless volatile liquid with the formula CS 2The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solventIt has an ether-like odor but commercial samples are typically contaminated with foul-smelling impurities. Carbon disulfide also spelled as carbon disulphide is a neurotoxic colorless volatile liquid with the formula CS 2The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solventIt has an ether-like odor but commercial samples are typically contaminated with foul-smelling impurities. In the food that we eat the clothes that we wear and even in the lead of the pencil by which we write. Its main use is in the manufacture of precursors to polyurethane dyes and other industrial chemicals. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.

It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Aniline is an organic compound with the formula C 6 H 5 NH 2Consisting of a phenyl group attached to an amino group aniline is the simplest aromatic amineIt is an industrially significant commodity chemical as well as a versatile starting material for fine chemical synthesis. The atomic number of carbon is 6 and the atomic mass is 1201gmol-1. Carbon is a member of the 14th group. We synthesized three kinds of sulfide polymers with different molar ratios.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass of carbon disulfide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.