Molar mass of chloroform
Molar Mass Of Chloroform. Suppose 26 g of calcium chloride CaCl2 11098 g mol are dissolved in water to make 200 mL of solution. It has the mass. The mass composition of a sample is 5267 of carbon 933 of hydrogen 682 of nitrogen and 3118 of oxygen. 967 C 1421 F.
 Calculate The Molecular Mass Of Chloroform Chcl 3 Atomic Masses C 12 U H 1 U Cl Youtube From youtube.com
Calculate The Molecular Mass Of Chloroform Chcl 3 Atomic Masses C 12 U H 1 U Cl Youtube From youtube.com
What is the density of the gas in gL-1. Practice Problems Problem 1. 31290 K at 760 mmHg. The vapor pressure of water at this temperature is 2602 torr. What is the density of the gas in gL-1. Buffers such as Na K phosphate and salts present a problem for ESI by lowering the vapor pressure of the droplets resulting in reduced signal through an increase in droplet surface tension.
Buffers such as Na K phosphate and salts present a problem for ESI by lowering the vapor pressure of the droplets resulting in reduced signal through an increase in droplet surface tension.
100 mol is to 09715 as x is to 00285 x 0029336 mol. It is miscible with water and with most common organic solvents. 100 mol is to 09715 as x is to 00285 x 0029336 mol. 396 C 1033 F. Molar Mass of Frequently Calculated Chemicals. 13266 gcm 3 20 C Melting point.
 Source: homeworklib.com
Source: homeworklib.com
31290 K at 760 mmHg. This compound is a cobalt complex. If the molar mass of the compound is 40304 g mol 1 the compound is magnesium oxide. Suppose 26 g of calcium chloride CaCl2 11098 g mol are dissolved in water to make 200 mL of solution. Practice Problems Problem 1.
 Source: youtube.com
Source: youtube.com
What is the density of the gas in gL-1. What is the density of the gas in gL-1. This compound is a cobalt complex. 31290 K at 760 mmHg. 8493 gmol 1 Appearance Colorless liquid Odor.
 Source: orginorgcompound.blogspot.com
Source: orginorgcompound.blogspot.com
256 gL 15 C 175 gL 25 C 158 gL 30 C 52 gL 60 C Solubility. N-Methyl-2-pyrrolidone NMP is an organic compound consisting of a 5-membered lactamIt is a colorless liquid although impure samples can appear yellow. 8493 gmol 1 Appearance Colorless liquid Odor. 3128 K decomposes at 720 C 3975 C 10355 F. Buffers such as Na K phosphate and salts present a problem for ESI by lowering the vapor pressure of the droplets resulting in reduced signal through an increase in droplet surface tension.
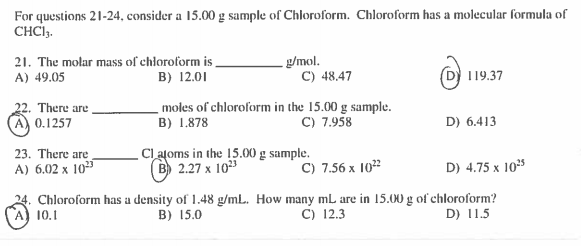 Source: chegg.com
Source: chegg.com
The 100 mole of chloroform represents a mole fraction of 09715. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. This compound is a cobalt complex. Buffers such as Na K phosphate and salts present a problem for ESI by lowering the vapor pressure of the droplets resulting in reduced signal through an increase in droplet surface tension. 967 C 1421 F.
 Source: slideplayer.com
Source: slideplayer.com
A molar volume of gas whose mass is 222gmol-1 is confined in a 224L container. Molar Mass of Frequently Calculated Chemicals. It is miscible with water and with most common organic solvents. What is the density of the gas in gL-1. To Determine the Empirical Formula.
 Source: slideplayer.com
Source: slideplayer.com
100 mol is to 09715 as x is to 00285 x 0029336 mol. 967 C 1421 F. A molar volume of gas whose mass is 222gmol-1 is confined in a 224L container. It is miscible with water and with most common organic solvents. The mass composition of a sample is 5267 of carbon 933 of hydrogen 682 of nitrogen and 3118 of oxygen.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
100 mol is to 09715 as x is to 00285 x 0029336 mol. Practice Problems Problem 1. What is the density of the gas in gL-1. 967 C 1421 F. This approach while less sensitive can be effective for otherwise insoluble compounds.
 Source: youtube.com
Source: youtube.com
It is miscible with water and with most common organic solvents. Suppose 26 g of calcium chloride CaCl2 11098 g mol are dissolved in water to make 200 mL of solution. A molar volume of gas whose mass is 222gmol-1 is confined in a 224L container. The mass composition of a sample is 5267 of carbon 933 of hydrogen 682 of nitrogen and 3118 of oxygen. It also belongs to the class of dipolar aprotic solvents such as dimethylformamide and dimethyl sulfoxideIt is used in the petrochemical and plastics industries as a.
 Source: youtube.com
Source: youtube.com
Practice Problems Problem 1. It has the mass. To Determine the Empirical Formula. 100 mol is to 09715 as x is to 00285 x 0029336 mol. 31290 K at 760 mmHg.
 Source: doubtnut.com
Source: doubtnut.com
3128 K decomposes at 720 C 3975 C 10355 F. If the vapor pressure of the solution is 2351 torr into how many ions does the electrolyte dissociate. To Determine the Empirical Formula. To Determine the Empirical Formula. If the molar mass of the compound is 40304 g mol 1 the compound is magnesium oxide.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass of chloroform by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






