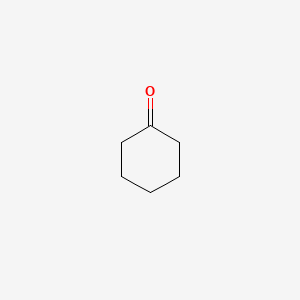
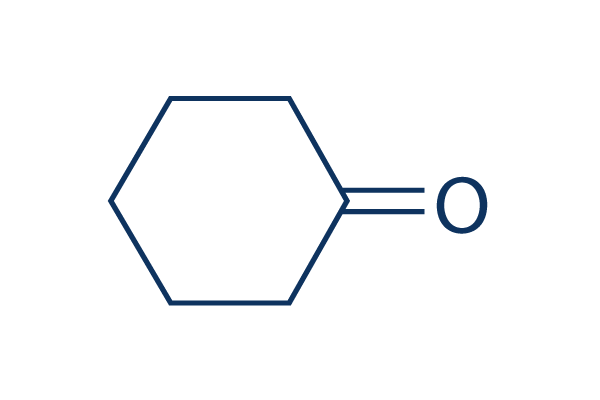
Molar mass of cyclohexanone
Molar Mass Of Cyclohexanone. 1697 K Boiling point. And due to the similarity in the terms many students have a hard time remembering which one is which or even know the difference. The catalytic upgrading of phenolic derivatives is vital for the efficient utilization of biomass resources. The set of calculations must also include the propagation of error if required.

Van der Waals suggested a modification to take into account molecular size and molecular interaction forces. 1697 K Boiling point. Range at 29815 30315 30815 and 31315 K and 01 MPa by means of a vibrating-tube densimeter. Miscible with organic solvents Vapor pressure. Cyclohexanone also known as oxocyclohexane pimelic ketone ketohexamethylene cyclohexyl ketone or ketocyclohexane is a six-carbon cyclic molecule with a ketone functional groupIt is a colorless oily liquid with an acetone-like smell. Slightly soluble in water Solubility.
1035 C 1543 F.
Three basic requirements must be met for explosion to take place. The ideal gas law treats the molecules of a gas as point particles with perfectly elastic collisions. Preparation of Phenol - Phenol was first mined from coal tar but today is manufactured on a large scale around 7 billion kgyear from petroleum. Three basic requirements must be met for explosion to take place. 893 kPa 20 C 119 kPa 25 C Henrys law constant k H 0022 molkg 1 bar 1. The Flammable Range also called Explosive Range is the concentration range of a gas or vapor that will burn or explode if an ignition source is introduced.

Flammable substance - fuel. 895 gml and the molar mass is 895 gmol. 8298 C 18136 F. Miscible with organic solvents Vapor pressure. Of cyclohexanone n-butylbenzene with neg.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The mixture was spread on a polypropylene PP. This colorless oil has an odor reminiscent of that of acetoneOver time samples of cyclohexanone assume a yellow color. 893 kPa 20 C 119 kPa 25 C Henrys law constant k H 0022 molkg 1 bar 1. Source of ignition - spark or high heat. VE data are sigmoid shape for the studied mixts.
Of cyclohexanone n-butylbenzene with neg. The mixture was spread on a polypropylene PP. 82143 gmol Appearance colorless liquid Odor. This works well for dilute gases in many experimental circumstances. Preparation of Phenol - Phenol was first mined from coal tar but today is manufactured on a large scale around 7 billion kgyear from petroleum.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Preparation of phenols from diazonium salts benzene sulphonic acid haloarenes cumene. Of cyclohexanone n-butylbenzene with neg. The set of calculations must also include the propagation of error if required. 82143 gmol Appearance colorless liquid Odor. LiTFSI was completely dissolved in DMF then the triblock polymer was added to the solution according to the molar ratio of EOLi 201 and the mixture was heated and stirred at 50 C for 24 h to obtain a yellow transparent solution.

Preparation of phenols from diazonium salts benzene sulphonic acid haloarenes cumene. However gas molecules are not point masses and there are many cases gases need to be treated as non-idealJohannes D. It is an important industrial product as a pioneer to various materials and useful compounds. They are known as carbolic acids. 895 gml and the molar mass is 895 gmol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Three basic requirements must be met for explosion to take place. Dry cyclohexanone was obtained by drying. 1035 C 1543 F. Preparation of phenols from diazonium salts benzene sulphonic acid haloarenes cumene. Oxidizer - oxygen or air.
 Source: molinstincts.com
Source: molinstincts.com
After the solvent evaporated the film was annealed. However gas molecules are not point masses and there are many cases gases need to be treated as non-idealJohannes D. They are known as carbolic acids. Dry cyclohexanone was obtained by drying. LiTFSI was completely dissolved in DMF then the triblock polymer was added to the solution according to the molar ratio of EOLi 201 and the mixture was heated and stirred at 50 C for 24 h to obtain a yellow transparent solution.
 Source: selleckchem.com
Source: selleckchem.com
Three basic requirements must be met for explosion to take place. The support spontaneous in situ redox method is employed to prepare the supported catalyst. This colorless oil has an odor reminiscent of that of acetoneOver time samples of cyclohexanone assume a yellow color. 895 gml and the molar mass is 895 gmol. The set of calculations must also include the propagation of error if required.
 Source: molinstincts.com
Source: molinstincts.com
VE values at lower mole fractions of cyclohexanone and neg. Van der Waals suggested a modification to take into account molecular size and molecular interaction forces. Preparation of Phenol - Phenol was first mined from coal tar but today is manufactured on a large scale around 7 billion kgyear from petroleum. LiTFSI was completely dissolved in DMF then the triblock polymer was added to the solution according to the molar ratio of EOLi 201 and the mixture was heated and stirred at 50 C for 24 h to obtain a yellow transparent solution. The mixture was spread on a polypropylene PP.

The Flammable Range also called Explosive Range is the concentration range of a gas or vapor that will burn or explode if an ignition source is introduced. LiTFSI was completely dissolved in DMF then the triblock polymer was added to the solution according to the molar ratio of EOLi 201 and the mixture was heated and stirred at 50 C for 24 h to obtain a yellow transparent solution. Of cyclohexanone n-butylbenzene with neg. 893 kPa 20 C 119 kPa 25 C Henrys law constant k H 0022 molkg 1 bar 1. Source of ignition - spark or high heat.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass of cyclohexanone by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.