Molar mass of difluoroethane
Molar Mass Of Difluoroethane. The experimental model was designed to mimic. 783 mmHg 20 C Acidity pK a 317 in water. 2926 K Solubility in water. When abused inhaled DFE produces intoxication and loss of muscular coordination.
 1 Chloro 1 1 Difluoroethane Wikipedia From en.wikipedia.org
1 Chloro 1 1 Difluoroethane Wikipedia From en.wikipedia.org
1 as pressure increases the excess molar volume is positive and a maximum is observed at the region of highest isothermal. To investigate DFE toxicokinetics groups n 3 of Sprague-Dawley rats were exposed to 30 s of 20 Lmin DFE. 195 C 671 F. Molecular weights can be found in the NIOSH Pocket Guide to Chemical Hazards chemical supplier lists the NIST Chemistry WebBook or other online databases. 255573 00001 58517 005 50356 020 59838 050 004 004 Some general trends can be identified in the excess molar volume plots. These data include the following.
Kinetic theory also gives a relation for the mean velocity v of molecules of mass m.
C2h2f2 isomers email protected. 783 mmHg 20 C Acidity pK a 317 in water. For properties that require the molar mass molecular weight for the conversion process the value of the molar mass must be included as the sixth input to the REFPROP routine. Temperature K A B C Reference Comment. Completely miscible liquid Vapor pressure. Calculate the molar mass of a vapor that has a density of 7130 gL at 13 C and 743 torr.
 Source: en.wikipedia.org
Source: en.wikipedia.org
When abused inhaled DFE produces intoxication and loss of muscular coordination. The experimental model was designed to mimic. 836 C 1185 F. The numeric value of 2445 in both formulae is the molar volume of air in litres at normal temperature and pressure NTP which is considered to be 25ºC and 1 atmosphere 101325 kPa or 760 mm Hg or 760 torr. Calculate the molar mass of a vapor that has a density of 7130 gL at 13 C and 743 torr.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Molecular weights can be found in the NIOSH Pocket Guide to Chemical Hazards chemical supplier lists the NIST Chemistry WebBook or other online databases. Das Reed et al 1973. 836 C 1185 F. Where η is in units of µPa s and M is the molar mass in gmol. 783 mmHg 20 C Acidity pK a 317 in water.
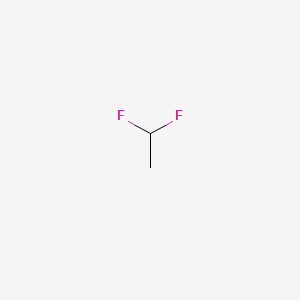
836 C 1185 F. 11-Difluoroethane DFE is a halogenated hydrocarbon used as a propellant in products designed for dusting electronic equipment and air brush painting. 115 gL gas 25 C 099 gmL liquid 195 C 1663 gmL solid 125 C Melting point. 836 C 1185 F. The experimental model was designed to mimic.
 Source: molinstincts.com
Source: molinstincts.com
When abused inhaled DFE produces intoxication and loss of muscular coordination. 115 gL gas 25 C 099 gmL liquid 195 C 1663 gmL solid 125 C Melting point. To investigate DFE toxicokinetics groups n 3 of Sprague-Dawley rats were exposed to 30 s of 20 Lmin DFE. Das Reed et al 1973. 11-Difluoroethane DFE is a halogenated hydrocarbon used as a propellant in products designed for dusting electronic equipment and air brush painting.
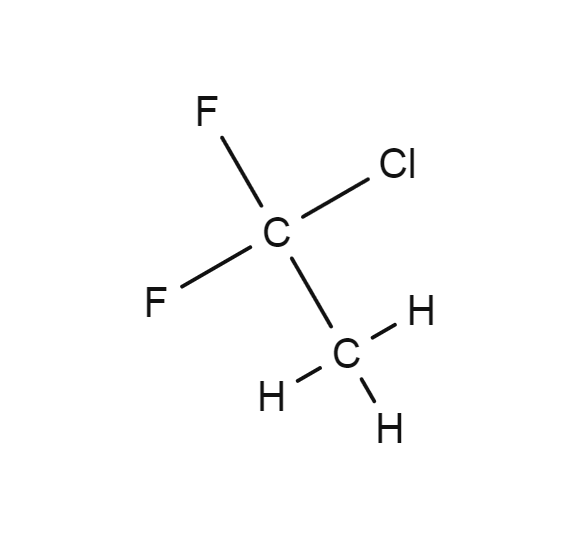 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Where η is in units of µPa s and M is the molar mass in gmol. Where η is in units of µPa s and M is the molar mass in gmol. The experimental model was designed to mimic. The numeric value of 2445 in both formulae is the molar volume of air in litres at normal temperature and pressure NTP which is considered to be 25ºC and 1 atmosphere 101325 kPa or 760 mm Hg or 760 torr. Coefficents calculated by NIST from authors data.
 Source: molinstincts.com
Source: molinstincts.com
Accurate thermophysical properties are available for several fluids. Molecular weights can be found in the NIOSH Pocket Guide to Chemical Hazards chemical supplier lists the NIST Chemistry WebBook or other online databases. 2926 K Solubility in water. The numeric value of 2445 in both formulae is the molar volume of air in litres at normal temperature and pressure NTP which is considered to be 25ºC and 1 atmosphere 101325 kPa or 760 mm Hg or 760 torr. Kinetic theory also gives a relation for the mean velocity v of molecules of mass m.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The experimental model was designed to mimic. Coefficents calculated by NIST from authors data. 783 mmHg 20 C Acidity pK a 317 in water. Accurate thermophysical properties are available for several fluids. For properties that require the molar mass molecular weight for the conversion process the value of the molar mass must be included as the sixth input to the REFPROP routine.
 Source: wikiwand.com
Source: wikiwand.com
Calculate the molar mass of a vapor that has a density of 7130 gL at 13 C and 743 torr. Gas Air Ar CO2 H2 He Kr N2 NH3 Ne O2 Xe. Das Reed et al 1973. C2h2f2 isomers email protected. To investigate DFE toxicokinetics groups n 3 of Sprague-Dawley rats were exposed to 30 s of 20 Lmin DFE.
 Source: molinstincts.com
Source: molinstincts.com
Calculate the molar mass of a vapor that has a density of 7130 gL at 13 C and 743 torr. Temperature K A B C Reference Comment. Where η is in units of µPa s and M is the molar mass in gmol. Instructions for Use of this Workbook and Converting 9x to 10 Exergy CExergy Flow exergy Closed system exergy 100001 1000 100070 1000 DREFPROPVBREFPROPXLS. 255573 00001 58517 005 50356 020 59838 050 004 004 Some general trends can be identified in the excess molar volume plots.
 Source: molinstincts.com
Source: molinstincts.com
2926 K Solubility in water. 1896 K Boiling point. Calculate the molar mass of a vapor that has a density of 7130 gL at 13 C and 743 torr. Thermal elimination of HCl from 1-chloro-11-difluoroethane is the principal industrial routedehydrohalogenation of 1-bromo-11-difluoroethane or 111-trifluoroethane or dehalogenation of 12-dichloro-11-difluoroethane are alternative routes. Kinetic theory also gives a relation for the mean velocity v of molecules of mass m.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass of difluoroethane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.