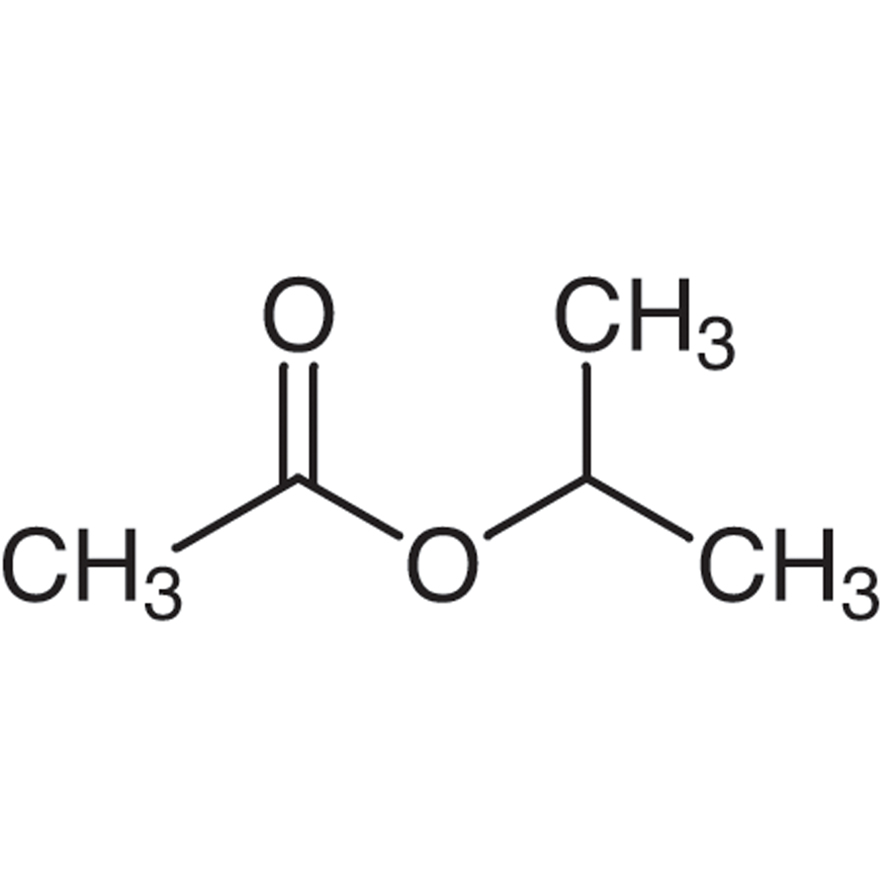
Molar mass of isopropyl acetate
Molar Mass Of Isopropyl Acetate. The mass of solute per 100 mL of solution is abbreviated as. We can use the molar mass to convert the grams of CoCl 2 2H 2 O to moles. The unit of molar mass in the SI system is kilogram per mole. The molar mass is simply the mass of one mole of substance ie.
 Isopropyl Acetate 3b A0036 Cymitquimica From cymitquimica.com
Isopropyl Acetate 3b A0036 Cymitquimica From cymitquimica.com
ISOPROPYL ALCOHOL RUBBING IPA ISOPROPANOL 70 91 99. The molar mass is simply the mass of one mole of substance ie. The mass of solute per 100 mL of solution is abbreviated as. Isopropyl alcohol is mixed with water to produce a 330 vv alcohol solution. Thymol also known as 2-isopropyl-5-methylphenol IPMP is a natural monoterpenoid phenol derivative of p-Cymene C 10 H 14 O isomeric with carvacrol found in oil of thyme and extracted from Thymus vulgaris common thyme ajwain and various other plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic propertiesThymol also provides the distinctive. The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number.
Converting the mass into moles.
The unit of molar mass in the SI system is kilogram per mole. Youll have to get up in the middle of the night to go to the bathroom and that will disturb your sleep. How many grams of sucrose are needed to make 775 mL of a 300 wv sucrose solution. Converting the mass into moles. 5 g of aluminum acetate. Limit 2 per order.
 Source: fishersci.co.uk
Source: fishersci.co.uk
They therefore have high boiling points compared to other substances of comparable molar mass. Converting the mass into moles. The acids with more than 10 carbon atoms are waxlike solids and their odor diminishes with increasing molar mass and resultant decreasing volatility. The carboxyl group readily engages in hydrogen bonding with water. The mass of solute per 100 mL of solution is abbreviated as.
Source: webbook.nist.gov
Amyl acetate pentyl acetate is an organic compound and an ester with the chemical formula CH 3 COOCH 2 4 CH 3 and the molecular weight 13019 gmol. Con acqua di rosa. Limit 2 per order. How many milliliters of each component are present in 705 mL of this solution. The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Limit 2 per order. The molar mass is simply the mass of one mole of substance ie. Con acqua di rosa. The carboxyl group readily engages in hydrogen bonding with water. Calculate the molarity of 0850 mol.
Source: chemspider.com
However esters formed from other pentanol isomers amyl alcohols or mixtures of pentanols. The mass of solute per 100 mL of solution is abbreviated as. Youll have to get up in the middle of the night to go to the bathroom and that will disturb your sleep. Asked 110717 isopropyl alcohol is mixed with water to produce a 37. 233 mL alcohol 472 mL water.
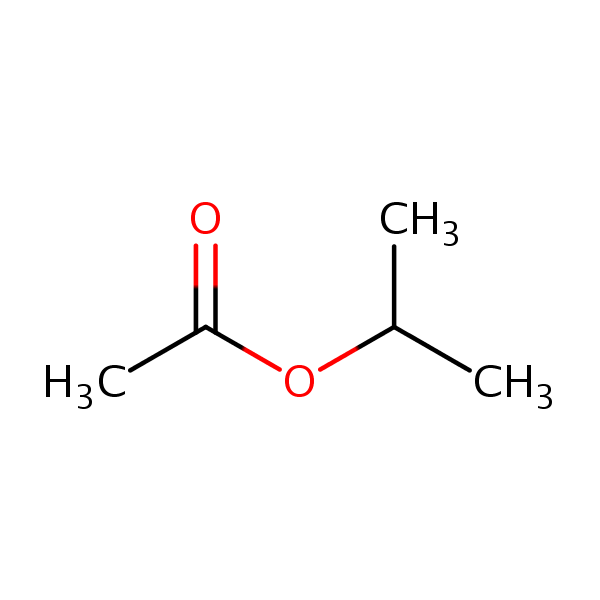 Source: sielc.com
Source: sielc.com
The acids with more than 10 carbon atoms are waxlike solids and their odor diminishes with increasing molar mass and resultant decreasing volatility. 233 mL alcohol 472 mL water. Calculate the molarity of 0850 mol. The Flammable Range also called Explosive Range is the concentration range of a gas or vapor that will burn or explode if an ignition source is introduced. The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The compound is the condensation product of acetic acid and 1-pentanol. The molar mass of CoCl 2 2H 2 O is 16587 gmol and includes the two water molecules as they are part of the crystal lattice structure of this solid hydrate 2. The acids with more than 10 carbon atoms are waxlike solids and their odor diminishes with increasing molar mass and resultant decreasing volatility. Three basic requirements must be met for explosion to take place. The unit of molar mass in the SI system is kilogram per mole.
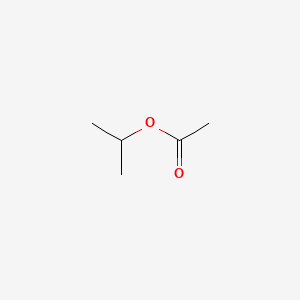
However esters formed from other pentanol isomers amyl alcohols or mixtures of pentanols. Converting the mass into moles. 233 mL alcohol 472 mL water. Carboxylic acids exhibit strong hydrogen bonding between molecules. Youll have to get up in the middle of the night to go to the bathroom and that will disturb your sleep.
 Source: en.wikipedia.org
Source: en.wikipedia.org
ISOPROPYL ALCOHOL RUBBING IPA ISOPROPANOL 70 91 99. Amyl acetate pentyl acetate is an organic compound and an ester with the chemical formula CH 3 COOCH 2 4 CH 3 and the molecular weight 13019 gmol. Source of ignition - spark or high heat. Flammable substance - fuel. Con acqua di rosa.
 Source: wikiwand.com
Source: wikiwand.com
They therefore have high boiling points compared to other substances of comparable molar mass. How many milliliters of each component are present in 705 mL of this solution. ISOPROPYL ALCOHOL RUBBING IPA ISOPROPANOL 70 91 99. Youll have to get up in the middle of the night to go to the bathroom and that will disturb your sleep. Isopropyl alcohol is mixed with water to produce a 330 vv alcohol solution.
 Source: cymitquimica.com
Source: cymitquimica.com
The mass of solute per 100 mL of solution is abbreviated as. Convert the volume into Liters. The molar mass of CoCl 2 2H 2 O is 16587 gmol and includes the two water molecules as they are part of the crystal lattice structure of this solid hydrate 2. The acids with more than 10 carbon atoms are waxlike solids and their odor diminishes with increasing molar mass and resultant decreasing volatility. How many grams of sucrose are needed to make 775 mL of a 300 wv sucrose solution.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass of isopropyl acetate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.