Molar mass of kcn
Molar Mass Of Kcn. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N. Molar mass 28534 gmol of moles of morphine of moles of HCl mol of HCl 00100 M x 000284 L 284x10-5 mol HCl 284x10-5 mol Morphine Mass of Morphine of moles x Molar mass 284x10-5 mol Morphine x 28534 gmol 81x10-3 g Morphine 81x10-3 g001 g 81 Answer. C 2 H 4 O.
 What Is The Molecular Formula And Molar Mass Of Potassium Cyanide Youtube From youtube.com
What Is The Molecular Formula And Molar Mass Of Potassium Cyanide Youtube From youtube.com
Find the molecular weight of the compound then divide the total mass of each element by it and multiply by 100. How many grams of chlorine are there in a 100-gram s. These data are consistent with the experimental values cited in the problem. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Your assessment is very important for improving the workof artificial intelligence which forms the content of this project. C 2 H 4 O.
In many weak aqueous solutions the molarity and molality are similar because one kilogram of water the solvent occupies one liter of volume at room temperature and the.
Molar Mass of Frequently Calculated Chemicals. Potassium permanganate is an inorganic compound with the chemical formula KMnO 4 and composed of K and MnO 4It is a purplish-black crystalline salt that dissolves in water to give intensely pink or purple solutions. One of the chlorofluorocarbons a freon has a molar mass of 1329 gmol and a percent composition by mass of 5334 Cl 2859 F and 1807 C. The reducing agent or reductant is an element that loses or donates electrons ie. It is a strong oxidizing agent and its most important application is in safety matches. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click Compute.
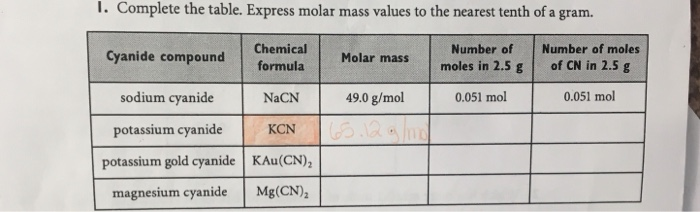 Source: chegg.com
Source: chegg.com
Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and. Sourcing 2021 new. Molar Mass of Frequently Calculated Chemicals. C 3 H 6 O. After sodium chlorate it is the second most common chlorate in industrial use.
 Source: clutchprep.com
Source: clutchprep.com
Determination of the reducing and oxidizing agents is coming in 2022. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and. Molar mass 28534 gmol of moles of morphine of moles of HCl mol of HCl 00100 M x 000284 L 284x10-5 mol HCl 284x10-5 mol Morphine Mass of Morphine of moles x Molar mass 284x10-5 mol Morphine x 28534 gmol 81x10-3 g Morphine 81x10-3 g001 g 81 Answer. Molality is based on mass so it can easily be converted into a mass ratio denoted by w.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Academiaedu is a platform for academics to share research papers. Molality is based on mass so it can easily be converted into a mass ratio denoted by w. Sourcing Guide for Brushless Rc Airplane Motors. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and. For C17H25N the molar mass 17C 25H 1N equals 24343 gmole and the three theoretical values for by weight are calculated as follows.
 Source: youtube.com
Source: youtube.com
C 2 H 4 O. Compared to molar concentration or mass concentration the preparation of a solution of a given molality is easy because it requires only a good scale. These data are consistent with the experimental values cited in the problem. For C20H25N3O the molar mass. Molar mass 28534 gmol of moles of morphine of moles of HCl mol of HCl 00100 M x 000284 L 284x10-5 mol HCl 284x10-5 mol Morphine Mass of Morphine of moles x Molar mass 284x10-5 mol Morphine x 28534 gmol 81x10-3 g Morphine 81x10-3 g001 g 81 Answer.
 Source: youtube.com
Source: youtube.com
Molar mass 28534 gmol of moles of morphine of moles of HCl mol of HCl 00100 M x 000284 L 284x10-5 mol HCl 284x10-5 mol Morphine Mass of Morphine of moles x Molar mass 284x10-5 mol Morphine x 28534 gmol 81x10-3 g Morphine 81x10-3 g001 g 81 Answer. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Find the molecular weight of the compound then divide the total mass of each element by it and multiply by 100. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. C 2 H 3 N.

Potassium chlorate is a compound containing potassium chlorine and oxygen with the molecular formula KClO 3In its pure form it is a white crystalline substance. A spontaneous decomposition of F3P results in a formation of 3-deoxyglucosone 3DG. Molar Mass of Frequently Calculated Chemicals. What is the reducing agent. In other applications it is mostly obsolete and has been.
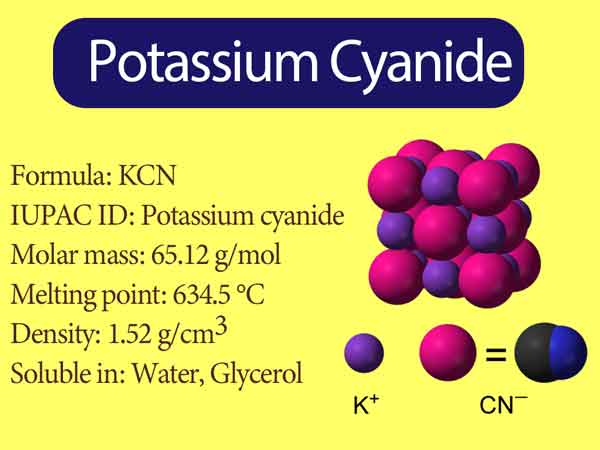 Source: chemistrypage.in
Source: chemistrypage.in
These data are consistent with the experimental values cited in the problem. One of the chlorofluorocarbons a freon has a molar mass of 1329 gmol and a percent composition by mass of 5334 Cl 2859 F and 1807 C. A spontaneous decomposition of F3P results in a formation of 3-deoxyglucosone 3DG. Compared to molar concentration or mass concentration the preparation of a solution of a given molality is easy because it requires only a good scale. LatexbM_solutefracn_solutem_solvent fracw_solutew_solventlatex Compared to molar concentration or mass concentration the preparation of a solution of a given molality is easy because it requires only a good scale.
The reducing agent or reductant is an element that loses or donates electrons ie. For C20H25N3O the molar mass. One of the chlorofluorocarbons a freon has a molar mass of 1329 gmol and a percent composition by mass of 5334 Cl 2859 F and 1807 C. Molality is based on mass so it can easily be converted into a mass ratio denoted by w. Determination of the reducing and oxidizing agents is coming in 2022.
 Source: youtube.com
Source: youtube.com
Academiaedu is a platform for academics to share research papers. C 2 H 4 O. It is a strong oxidizing agent and its most important application is in safety matches. Academiaedu is a platform for academics to share research papers. One of the chlorofluorocarbons a freon has a molar mass of 1329 gmol and a percent composition by mass of 5334 Cl 2859 F and 1807 C.
 Source: clutchprep.com
Source: clutchprep.com
C 2 H 3 N. In many weak aqueous solutions the molarity and molality are similar because one kilogram of water the solvent occupies one liter of volume at room temperature and the. 1 CrF 3 Cr. Potassium chlorate is a compound containing potassium chlorine and oxygen with the molecular formula KClO 3In its pure form it is a white crystalline substance. One of the chlorofluorocarbons a freon has a molar mass of 1329 gmol and a percent composition by mass of 5334 Cl 2859 F and 1807 C.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass of kcn by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.