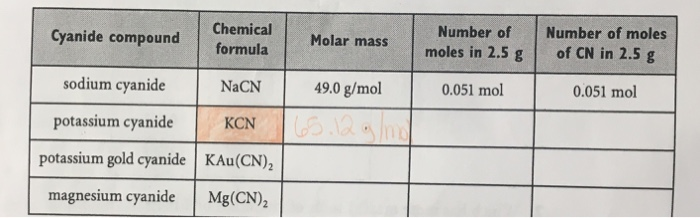
Molar mass of sodium cyanide
Molar Mass Of Sodium Cyanide. It has a white crystalline appearance as a solid and is odourless. What is the mass solubility of calcium sulfate in pure water expressed in gL. If the molar mass of the salt is 218 gmol what mass is required. We recommend you bookmark it so you can refer back to it.
C 2 H 3 N. The more commonly available pentahydrate from Na 2 S 2 O 35H 2 O has a molar mass of 24818 gmol. It has a faintly pungent smell similar to SO 2. 170 o C but begins to decompose at 150 o C. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml. We recommend you bookmark it so you can refer back to it.
Will precipitation occur when you add 005 mL of 010 M KBr to a saturated solution of AgCl.
What is the molar solubility of calcium sulfate in pure water. What is the molar solubility of calcium sulfate in pure. The more commonly available pentahydrate from Na 2 S 2 O 35H 2 O has a molar mass of 24818 gmol. Use the value ksp14x10-8 for PbI2 to solve the following problems. What is the mass solubility of calcium sulfate in pure water expressed in gL. The solubility of sodium thiosulfate in water is 70.

Molar Mass of Sodium carbonate Na2CO3 Molar Mass of Sodium nitrate NaNO3 Molar Mass of Sulfurous acid H2SO3 Molar Mass of Zinc sulfate ZnSO4 Bookmarking Save and Share Results. What is the molar solubility of calcium sulfate in pure. It has a white crystalline appearance as a solid and is odourless. In terms of atomic masses. The tool is designed so you can flip between different parts of a problem set.
 Source: youtube.com
Source: youtube.com
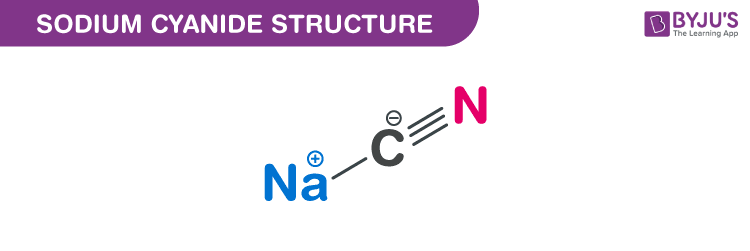
In a total of 14204 g of sodium sulfate 4598 g are sodium 3206 g are sulfur and 6400 g are oxygen. In a total of 14202 parts by mass of sodium sulfate 4598 parts are sodium 3206 parts are sulfur and 6400 parts are oxygen. Molar Mass of Frequently Calculated Chemicals. In terms of a gram mass unit molar mass. Sodium cyanide is a poisonous compound with the formula Na C NIt is a white water-soluble solid.
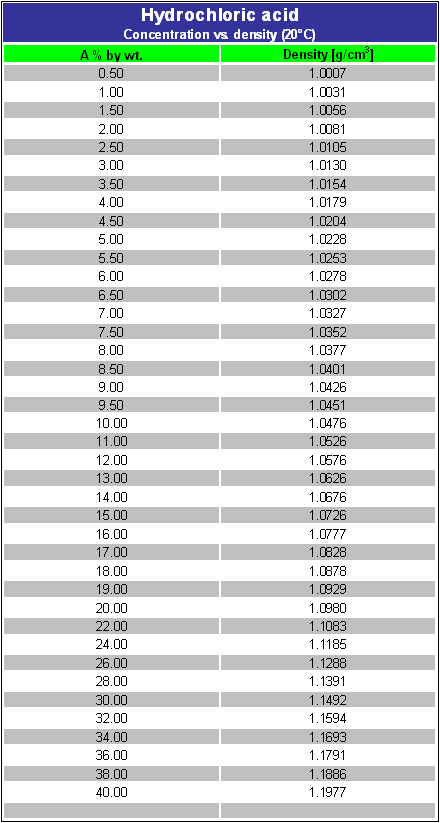
How many grams of H_3PO_4. C 2 H 3 N. In a total of 14204 g of sodium sulfate 4598 g are sodium 3206 g are sulfur and 6400 g are oxygen. Molar mass gmol Density Range of concentration. Its main application in gold mining also exploits its high reactivity toward metalsIt is a moderately strong baseWhen treated with acid it forms the toxic gas hydrogen cyanide.
 Source: youfriendjili.com
Source: youfriendjili.com
190107 grams per mole. C 2 H 5 NO. Sodium sulfite has a white or whitish-yellow appearance in its solid-state. What is the mass solubility of calcium sulfate in pure water expressed in gL. The more commonly available pentahydrate from Na 2 S 2 O 35H 2 O has a molar mass of 24818 gmol.
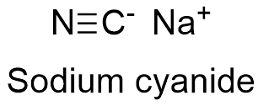 Source: softschools.com
Source: softschools.com
Na 2 S 2 O 5. The experiment described above is repeated using 500 mL of 10 mol L-1 sodium hydroxide a strong monobasic base and 10 mol L-1 hydrogren cyanide HCN a weak monoprotic acid K a 6 10-10 instead of 10 mol L-1 hydrochloric acid a strong monoprotic acid. 190107 grams per mole. What volume of 12 M HCl solution is needed to prepare 5 liters of 00250 M solution. Na 2 S 2 O 5.
 Source: en.wikipedia.org
Source: en.wikipedia.org
What is the molar solubility of calcium sulfate in pure water. The experiment described above is repeated using 500 mL of 10 mol L-1 sodium hydroxide a strong monobasic base and 10 mol L-1 hydrogren cyanide HCN a weak monoprotic acid K a 6 10-10 instead of 10 mol L-1 hydrochloric acid a strong monoprotic acid. Molar mass gmol Density Range of concentration. The pentahydrate of this salt has a melting point of 3214 K and a boiling point of 373 K. The solubility of sodium thiosulfate in water is 70.

The solubility of sodium thiosulfate in water is 70. What is the molar solubility of calcium sulfate in pure. What is the mass solubility of calcium sulfate in pure water expressed in gL. In terms of atomic masses. The density of sodium thiosulfate corresponds to 1667 grams per cubic centimetres.
 Source: chegg.com
Source: chegg.com
The pentahydrate of this salt has a melting point of 3214 K and a boiling point of 373 K. 170 o C but begins to decompose at 150 o C. Cyanide has a high affinity for metals which leads to the high toxicity of this salt. What is the molar solubility of calcium sulfate in pure water. It has a faintly pungent smell similar to SO 2.
 Source: byjus.com
Source: byjus.com
C 2 H 5 NO. In a total of 14204 g of sodium sulfate 4598 g are sodium 3206 g are sulfur and 6400 g are oxygen. 170 o C but begins to decompose at 150 o C. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Na 2 S 2 O 5.
 Source: en.wikipedia.org
Source: en.wikipedia.org
C 2 H 5 NO. In a total of 14202 parts by mass of sodium sulfate 4598 parts are sodium 3206 parts are sulfur and 6400 parts are oxygen. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. In terms of a gram mass unit molar mass. How many grams of H_3PO_4.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass of sodium cyanide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.