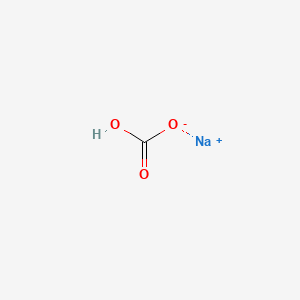
Molar mass of sodium hydrogen carbonate
Molar Mass Of Sodium Hydrogen Carbonate. As per the periodic table relative atomic mass of Sodium Na 2299 and Chlorine Cl 3545. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. Atomic mass the mass of an atom expressed in atomic mass units amu. What is the molarity of sulfuric acid if 4390 mL of H 2 SO 4 is required to neutralize 0455 g of sodium hydrogen carbonate.
Atomic number the number of protons in the nucleus of an atom. 1 x 120 12. A standard acid-base reaction occurs as sodium bicarbonate aka baking soda is a weak base. Due to this sodium is a highly reactive chemical element and is found only in the form of compounds. Molar Mass Is 9896G. The molecular weight would be.
Molar Mass Is 9896G.
Sodium percarbonate is a chemical substance with formula Na 2 H 3 CO 6It is an adduct of sodium carbonate soda ash or washing soda and hydrogen peroxide that is a perhydrate whose formula is more properly written as 2 Na 2 CO 3 3 H 2 O 2It is a colorless crystalline hygroscopic and water-soluble solid. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. Molar Mass Is 9896G. In other words the molar mass is the total mass of all the atoms in grams that make a mole of a particular molecule. What is the molarity of sulfuric acid if 4390 mL of H 2 SO 4 is required to neutralize 0455 g of sodium hydrogen carbonate. Grams mole molar mass.
 Source: selfstudy365.com
Source: selfstudy365.com
The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. As the atomic number of sodium suggests it has only one electron in its outermost orbit or its valency is 1. Chemically sodium is represented by the symbol Na. Computing Molecular Mass for a Covalent Compound Ibuprofen C 13 H 18 O 2 is a covalent compound and the active ingredient in several popular nonprescription pain medications such as Advil and MotrinWhat is the molecular mass amu for this compound. What is the molarity of sulfuric acid if 4390 mL of H 2 SO 4 is required to neutralize 0455 g of sodium hydrogen carbonate.
 Source: slideplayer.com
Source: slideplayer.com
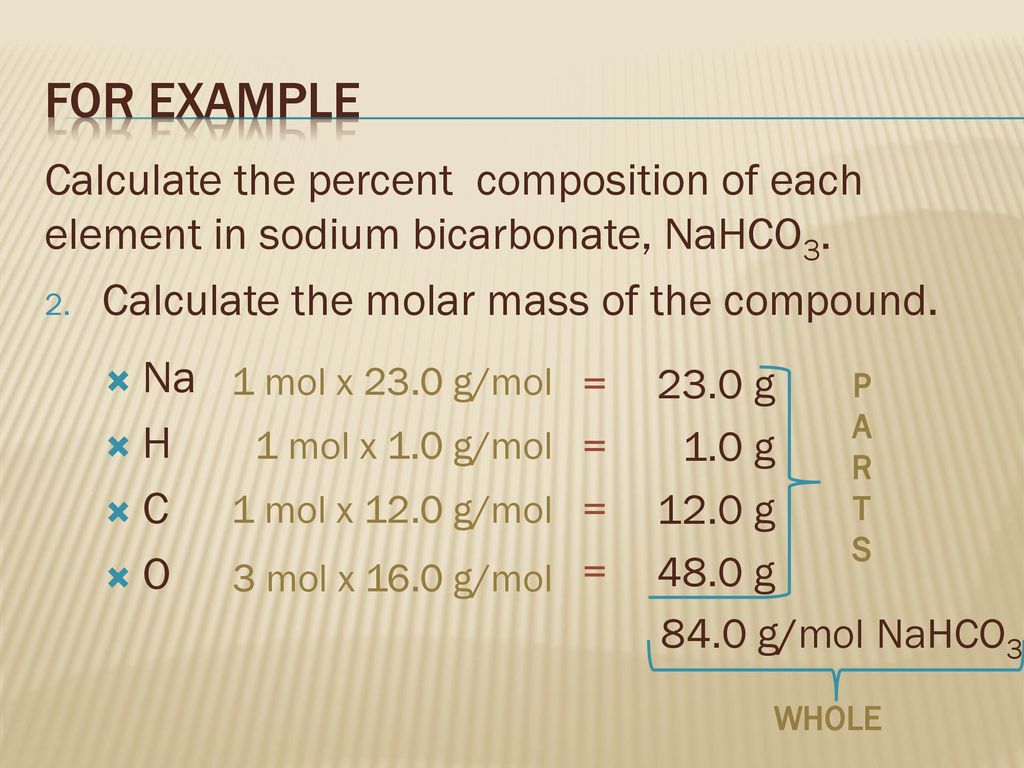
In other words the molar mass is the total mass of all the atoms in grams that make a mole of a particular molecule. Chlorine7165 355 201. Hydrogen 407 1 407. The molecular weight would be. Youll get an aqueous solution of the salt sodium chloride in this case water and carbon dioxide gas given off as bubbles out of the solution.
 Source: youtube.com
Source: youtube.com
Since sodium carbonate contains two atoms of sodium one atom of carbon and three atoms of oxygen. Atomic mass the mass of an atom expressed in atomic mass units amu. Hydrogen 407 201 2. Now we have to divide all the values with the lowest obtained value. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass.
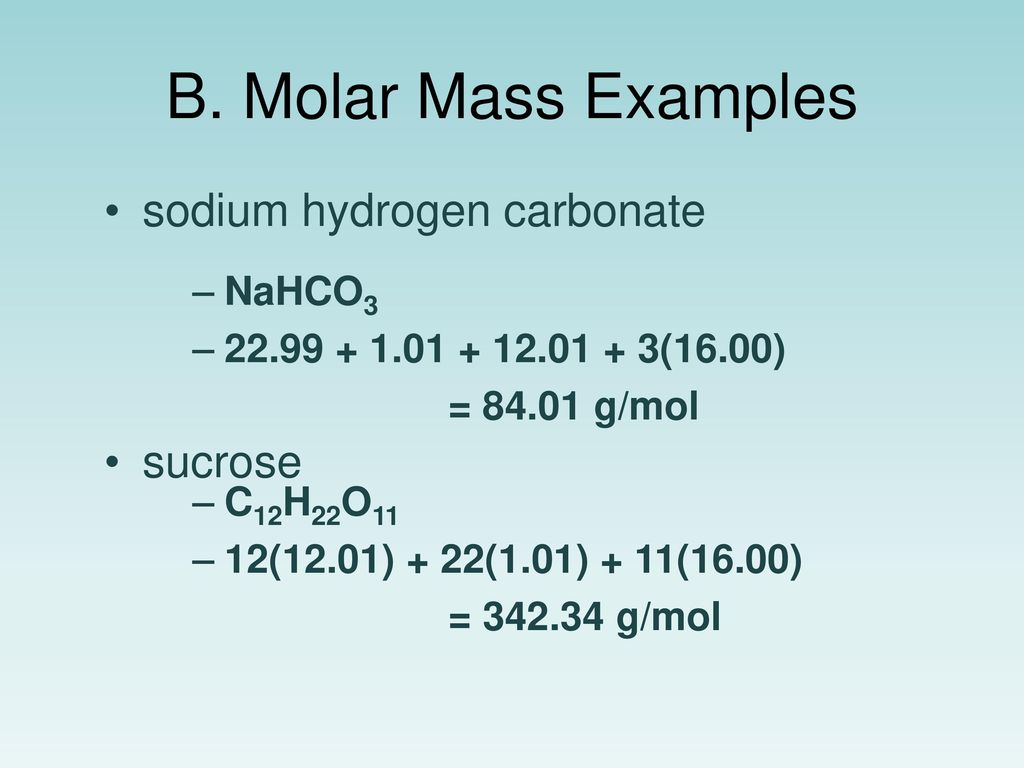 Source: slideplayer.com
Source: slideplayer.com
Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Solution Molecules of this compound are comprised of 13 carbon atoms 18 hydrogen atoms and 2 oxygen atoms. To Calculate Empirical Formulae first we have to divide the given percentages of atoms by their molecular masses. The total number of protons and neutrons in the nucleus. 2 x 230 46.

Now we have to divide all the values with the lowest obtained value. Now we have to divide all the values with the lowest obtained value. It is sometimes abbreviated as SPCIt contains 325 by weight of hydrogen. Hydrogen 407 1 407. Grams 58443 5 292215 g.
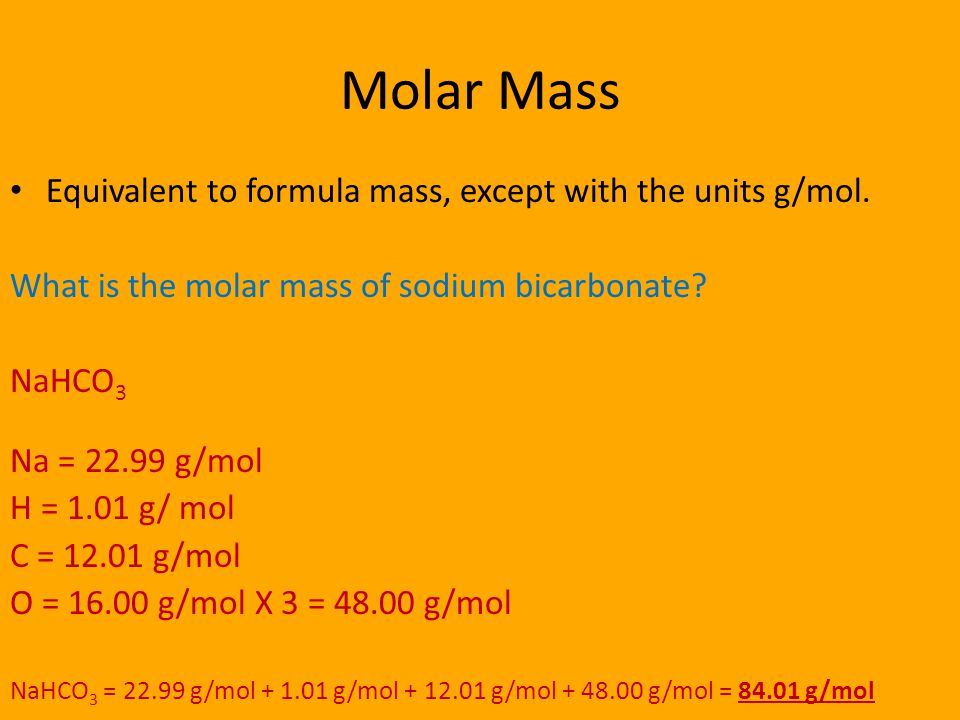 Source: slideplayer.com
Source: slideplayer.com
Atomic number of sodium is 11 and its atomic mass is 2298. Answer 1 of 4. Sodium percarbonate is a chemical substance with formula Na 2 H 3 CO 6It is an adduct of sodium carbonate soda ash or washing soda and hydrogen peroxide that is a perhydrate whose formula is more properly written as 2 Na 2 CO 3 3 H 2 O 2It is a colorless crystalline hygroscopic and water-soluble solid. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. There are 20 known isotopes of sodium but only one.
 Source: slideplayer.com
Source: slideplayer.com
7798 gmol Appearance yellow to white powder Density. Grams 58443 5 292215 g. In other words the molar mass is the total mass of all the atoms in grams that make a mole of a particular molecule. As per the periodic table relative atomic mass of Sodium Na 2299 and Chlorine Cl 3545. Computing Molecular Mass for a Covalent Compound Ibuprofen C 13 H 18 O 2 is a covalent compound and the active ingredient in several popular nonprescription pain medications such as Advil and MotrinWhat is the molecular mass amu for this compound.
 Source: youtube.com
Source: youtube.com
If 1 kg of pure copper contains 1574 Moles what is the molar mass of. Grams 58443 5 292215 g. Sodium percarbonate is a chemical substance with formula Na 2 H 3 CO 6It is an adduct of sodium carbonate soda ash or washing soda and hydrogen peroxide that is a perhydrate whose formula is more properly written as 2 Na 2 CO 3 3 H 2 O 2It is a colorless crystalline hygroscopic and water-soluble solid. 7798 gmol Appearance yellow to white powder Density. Since sodium carbonate contains two atoms of sodium one atom of carbon and three atoms of oxygen.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
H 2 SO 4 aq 2NaHCO 3 aq — Na 2 SO 4 aq 2CO 2 g 2H 2 Oℓ The key ratio is that 1 mole of sulfuric acid is needed for every 2 moles of. 4 Na O 2 2 Na 2 O 2 Na 2 O O 2. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. H 2 SO 4 aq 2NaHCO 3 aq — Na 2 SO 4 aq 2CO 2 g 2H 2 Oℓ The key ratio is that 1 mole of sulfuric acid is needed for every 2 moles of. Grams mole molar mass.
 Source: slideplayer.com
Source: slideplayer.com
What is the molarity of sulfuric acid if 4390 mL of H 2 SO 4 is required to neutralize 0455 g of sodium hydrogen carbonate. Chlorine7165 355 201. 7798 gmol Appearance yellow to white powder Density. Grams 58443 5 292215 g. Atomic number of sodium is 11 and its atomic mass is 2298.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass of sodium hydrogen carbonate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.