Molar mass of sodium iodide
Molar Mass Of Sodium Iodide. For example the solubility of the artists pigment chrome yellow PbCrO 4 is 46 10 6 gL. NickelII chloride in various forms is the most important source of nickel for chemical synthesis. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. Molar Mass of Frequently Calculated Chemicals.
 How To Balance Na I2 Nai Sodium Iodine Gas Youtube From youtube.com
How To Balance Na I2 Nai Sodium Iodine Gas Youtube From youtube.com
What mass of sodium iodide is required to prepare a 2500mL volume of 150molL-1 concentration with respect to the salt. 7295 JmolK Hydrogen Bond Acceptor. Mass in the floor of the mouth of an African American smoker. The nickel chlorides are deliquescent absorbing moisture from the air to form a solution. Chemical Properties of Sodium Oxide Na 2 O. The given mass of K 47 g is a bit more than one-tenth the molar mass 3910 g so a reasonable ballpark estimate of the number of moles would be slightly greater than 01 mol.
What mass of sodium iodide is required to prepare a 2500mL volume of 150molL-1 concentration with respect to the salt.
For example the solubility of the artists pigment chrome yellow PbCrO 4 is 46 10 6 gL. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. Determine the solubility product for PbCrO 4. Even though a sodium cation has a slightly smaller mass than a sodium atom since it is missing an electron this difference will be offset by the fact that a chloride anion is slightly more massive than a chloride atom due to the extra electron. 3 is 1401 amu 3101amu 1704 amu. Molecular Weight Molar Mass.
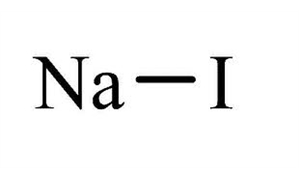 Source: lobachemie.com
Source: lobachemie.com
Reacts with water and ethanol. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. Mass in the floor of the mouth of an African American smoker. Question 5bc2b Question 3917f. Molar Mass of Frequently Calculated Chemicals.

For example the solubility of the artists pigment chrome yellow PbCrO 4 is 46 10 6 gL. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. Molecular Weight Molar Mass. Oral Surgery Oral Medicine Oral Pathology and Oral Radiology. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when.
 Source: byjus.com
Source: byjus.com
NickelII chloride in various forms is the most important source of nickel for chemical synthesis. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. Determination of K sp from Gram Solubility Many of the pigments used by artists in oil-based paints Figure 153 are sparingly soluble in water. Mass in the floor of the mouth of an African American smoker. A slow-growing anterior maxillary mass.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Moreover the mass of an electron is negligibly small with respect to the mass of a typical atom. 7295 JmolK Hydrogen Bond Acceptor. Oral Surgery Oral Medicine Oral Pathology and Oral Radiology. Determine the solubility product for PbCrO 4. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol.
 Source: youtube.com
Source: youtube.com
NickelII chloride or just nickel chloride is the chemical compound NiCl 2The anhydrous salt is yellow but the more familiar hydrate NiCl 2 6H 2 O is green. Calculate the molar solubility of leadII iodide. What mass of sodium iodide is required to prepare a 2500mL volume of 150molL-1 concentration with respect to the salt. Mass in the floor of the mouth of an African American smoker. Question 5bc2b Question 3917f.
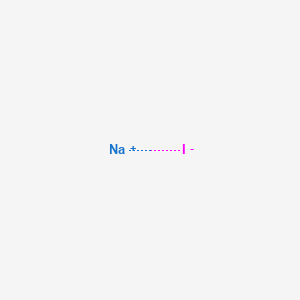
Physical Properties of Sodium Oxide Na 2 O. Physical Properties of Sodium Oxide Na 2 O. Chemical Properties of Sodium Oxide Na 2 O. Question 5bc2b Question 3917f. For example the solubility of the artists pigment chrome yellow PbCrO 4 is 46 10 6 gL.
 Source: youtube.com
Source: youtube.com
B The sum of the atomic masses for NH. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. Oral Surgery Oral Medicine Oral Pathology Oral Radiology is required reading for. What mass of sodium iodide is required to prepare a 2500mL volume of 150molL-1 concentration with respect to the salt. Chemical Properties of Sodium Oxide Na 2 O.
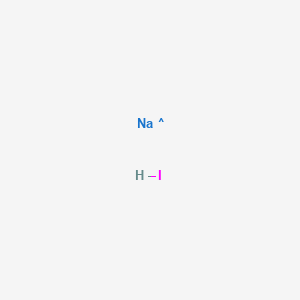
The molar mass equals the sum of the atomic masses expressed in gmol. The molar mass equals the sum of the atomic masses expressed in gmol. Suppose in an experiment to determine the amount of sodium hypochlorite in bleach 00000524 mol KIO3 were titrated with an unknown solution of Na2S2O3 and the endpoint was reached after 1780 mL How many moles of Na2S2O3 did this require. What mass of sodium iodide is required to prepare a 2500mL volume of 150molL-1 concentration with respect to the salt. Physical Properties of Sodium Oxide Na 2 O.
 Source: youtube.com
Source: youtube.com
Mass in the floor of the mouth of an African American smoker. B The sum of the atomic masses for NH. The molar mass equals the sum of the atomic masses expressed in gmol. When determining the amount of an oxidant present by titration you can use iodine and starch as an indicator. Referring to the periodic table the atomic mass of K is 3910 amu and so its molar mass is 3910 gmol.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. Oral Surgery Oral Medicine Oral Pathology and Oral Radiology. 15 10 3 M. Question 5bc2b Question 3917f. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass of sodium iodide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






