Molar mass of sodium iodide formula
Molar Mass Of Sodium Iodide Formula. Salt of Potassium Sodium and Ammonium. What mass of sodium iodide is required to prepare a 2500mL volume of 150molL-1 concentration with respect to the salt. This relationship holds for all elements since their atomic masses are measured relative to that of the amu-reference substance 12 C. In its anhydrous form sodium thiosulfate has a molar mass of 15811 grams per mole.
 Sodium Iodide Cas 7681 82 5 Scbt Santa Cruz Biotechnology From scbt.com
Sodium Iodide Cas 7681 82 5 Scbt Santa Cruz Biotechnology From scbt.com
The molar mass equals the sum of the atomic masses expressed in gmol. Sodium sulfate Na 2 SO 4. We provide solutions to students. This metal peroxide exists in several hydrates and peroxyhydrates including Na 2 O 2 2H 2 O 2 4H 2 O Na 2 O 2 2H 2 O Na 2 O 2 2H 2 O 2 and Na 2 O 2 8H 2 O. We begin by finding the atomic mass of each element in the periodic table. Chemical formula plays an important role in understanding different concepts of chemistry.
We provide solutions to students.
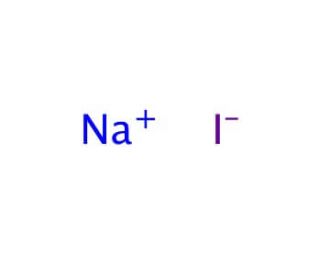
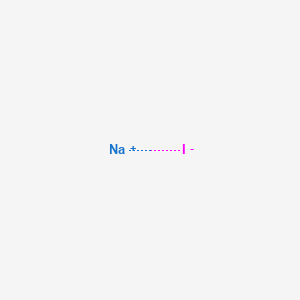
In its anhydrous form sodium thiosulfate has a molar mass of 15811 grams per mole. Salt of non-Potassium Sodium and Ammonium. Salt of Potassium Sodium and Ammonium. Determine the empirical formula of the metal iodide. We provide solutions to students. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodine.
 Source: byjus.com
Source: byjus.com
This relationship holds for all elements since their atomic masses are measured relative to that of the amu-reference substance 12 C. B the chemical formula of the compound appears after the arrow. A student found that 032 g of vapor had a volume of 152 mL at 100 degrees C and 1 atm. Salt of Potassium Sodium and Ammonium. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.
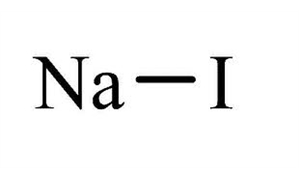 Source: lobachemie.com
Source: lobachemie.com
The density of sodium thiosulfate corresponds to 1667 grams per cubic centimetres. A the anesthetic halothane C 2 HBrClF 3. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. The more commonly available pentahydrate from Na 2 S 2 O 35H 2 O has a molar mass of 24818 gmol. It is a strong base.
Acid Metal Carbonate Salt Water Carbon. It is used mainly as a. A mole of 12 C weighs 12 g its molar mass is 12 gmol. Please Use Our Service If Youre. Acid Metal Carbonate Salt Water Carbon.
 Source: youtube.com
Source: youtube.com
This relationship holds for all elements since their atomic masses are measured relative to that of the amu-reference substance 12 C. Wishing for a unique insight into a subject matter for your subsequent individual research. Acid Metal Oxide Salt Water. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
 Source: youtube.com
Source: youtube.com
Wishing for a unique insight into a subject matter for your subsequent individual research. Extending this principle the molar mass of a compound in grams is likewise numerically equivalent to. A The atomic mass of Ag is 10787 amu and the molar mass of silver equals 10787 gmol. Potassium carbonate K 2 CO 3 2. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.
 Source: byjus.com
Source: byjus.com
It has a white crystalline appearance as a solid and is odourless. We provide solutions to students. Sodium sulfate Na 2 SO 4. Covalent compounds are composed of non-metallic elements. B the chemical formula of the compound appears after the arrow.
 Source: scbt.com
Source: scbt.com
We begin by finding the atomic mass of each element in the periodic table. Covalent compounds are composed of non-metallic elements. Calculate the empirical or molecular formula mass and the molar mass of each of the following minerals. B the chemical formula of the compound appears after the arrow. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodine.

Well we know that at STPStandard temperature27315 Kelvins and pressure1 atmospheric pressure any gas will have a volume of 224 Liters so now depending on the density of your compound or molecules we can reverse calculate the answer of. Please Use Our Service If Youre. Well we know that at STPStandard temperature27315 Kelvins and pressure1 atmospheric pressure any gas will have a volume of 224 Liters so now depending on the density of your compound or molecules we can reverse calculate the answer of. Salt of non-Potassium Sodium and Ammonium. This relationship holds for all elements since their atomic masses are measured relative to that of the amu-reference substance 12 C.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Potassium carbonate K 2 CO 3 2. This relationship holds for all elements since their atomic masses are measured relative to that of the amu-reference substance 12 C. Question 5bc2b Question 3917f. Wishing for a unique insight into a subject matter for your subsequent individual research. In its anhydrous form sodium thiosulfate has a molar mass of 15811 grams per mole.
This metal peroxide exists in several hydrates and peroxyhydrates including Na 2 O 2 2H 2 O 2 4H 2 O Na 2 O 2 2H 2 O Na 2 O 2 2H 2 O 2 and Na 2 O 2 8H 2 O. Answer 1 of 3. The density of sodium thiosulfate corresponds to 1667 grams per cubic centimetres. We provide solutions to students. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass of sodium iodide formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.