Molar mass of sulfur tetrafluoride
Molar Mass Of Sulfur Tetrafluoride. For a molecule with the. Van der Waals equation describes fluids composed of particles that are attracted to each other and have a non-negligible volume. Reacts with water Solubility. Molar mass of CO2 gmol 1201 gmol 2 x 1600 gmol O 4401 gmol CO2 The molar mass of ionic compounds is referred to as the formula mass because ionic.
 Oneclass What Is The Molar Mass Of Sulfur Tetrafluoride From oneclass.com
Oneclass What Is The Molar Mass Of Sulfur Tetrafluoride From oneclass.com
MgO is the other. B The number of moles and the mass of oxygen formed by the decomposition of 1252 g of silverI oxide. Reacts with ethanol soluble vague in acetonitrile. This book is wrote by Stanley E. An example is sulfur hexafluoride SF6 for which writing a Lewis structure with six SF bonds requires that at least 12 electrons be present around the sulfur atom. The density of sulfur hexafluoride is relatively high at room temperature and pressure due to the gass large molar mass.
Reacts with water Solubility.
If you want to cite. The electron-domain geometry of a sulfur-centered compound is trigonal bipyramidal. 303 to 305 K at 3 mmHg Solubility in water. What is the volume of 520g of gaseous silicon tetrafluoride SiF4 at STP. Unlike helium which has a molar mass of about 4 gmol and pitches the voice up SF 6 has a molar mass of about 146 gmol and the speed of sound through the gas is about 134 ms at room temperature pitching the voice down. AX 2 E 3 has linear shape.
 Source: youtube.com
Source: youtube.com
30 to 32 C 86 to 90 F. This book is wrote by Stanley E. The two Cl atoms also. CH3CCH IN LEWIS DOT DIAGRAMS FNON2H4PCL5CH3OHOH-LiOHNO-2NO2HNO3CH3CHOIF3Asf3SO2-3 H2SO4XeO3 Dot diagrams are almost impossible here in text with hard carriage returns. Aug 21 2021 XeF4 Molecular Geometry And Bond Angles In order to achieve this the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite 180 degree from each other.
If you are search for Coh2. Molar mass of CO2 gmol 1201 gmol 2 x 1600 gmol O 4401 gmol CO2 The molar mass of ionic compounds is referred to as the formula mass because ionic. Van der Waals equation describes fluids composed of particles that are attracted to each other and have a non-negligible volume. Corrosive flammable can be explosive GHS pictograms. An example is sulfur hexafluoride SF6 for which writing a Lewis structure with six SF bonds requires that at least 12 electrons be present around the sulfur atom.
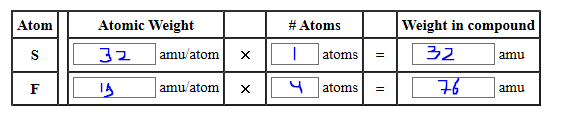 Source: bartleby.com
Source: bartleby.com
16119 gmol 1 Appearance colourless oil Density. Corrosive flammable can be explosive GHS pictograms. Molar mass of CO2 gmol 1201 gmol 2 x 1600 gmol O 4401 gmol CO2 The molar mass of ionic compounds is referred to as the formula mass because ionic. The F-S-F bond angles in SF6 are _____. Reacts with ethanol soluble vague in acetonitrile.
 Source: molinstincts.com
Source: molinstincts.com
Molar mass of CO2 gmol 1201 gmol 2 x 1600 gmol O 4401 gmol CO2 The molar mass of ionic compounds is referred to as the formula mass because ionic. H226 H302 H312. If you want to cite. We would like to show you a description here but the site wont allow us. Reacts with ethanol soluble vague in acetonitrile.
 Source: youtube.com
Source: youtube.com
If the temperature is 35C what. A gas with a molar mass of 2886gmol occupies 150 L of space at 250C and 200. If you are search for Coh2. C The number of moles and the mass of magnesium carbonate MgCO 3 required to produce 283 g of carbon dioxide. The pairwise attraction is called van der Walls force.
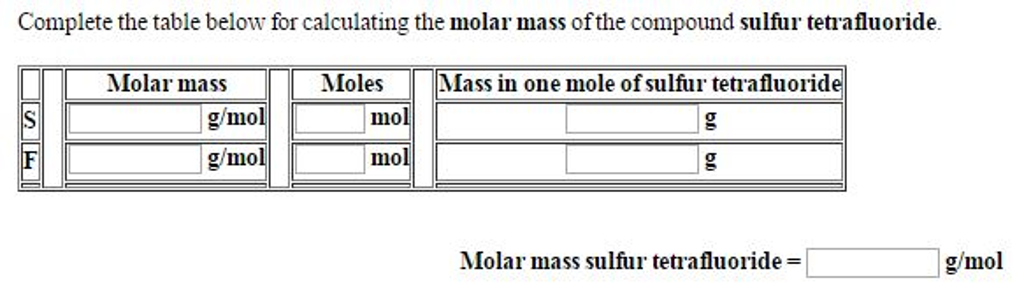 Source: chegg.com
Source: chegg.com
16119 gmol 1 Appearance colourless oil Density. An example is sulfur hexafluoride SF6 for which writing a Lewis structure with six SF bonds requires that at least 12 electrons be present around the sulfur atom. The pairwise attraction is called van der Walls force. The arrangement of the molecules in this compound is such that the Carbon atom is in the central atom one Hydrogen atom is on the upper topmost position and the other one is on Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. 303 to 305 K at 3 mmHg Solubility in water.
 Source: oneclass.com
Source: oneclass.com
Molar mass of CO2 gmol 1201 gmol 2 x 1600 gmol O 4401 gmol CO2 The molar mass of ionic compounds is referred to as the formula mass because ionic. A gas with a molar mass of 2886gmol occupies 150 L of space at 250C and 200. H226 H302 H312. Unlike helium which has a molar mass of about 4 gmol and pitches the voice up SF 6 has a molar mass of about 146 gmol and the speed of sound through the gas is about 134 ms at room temperature pitching the voice down. If the temperature is 35C what.

H226 H302 H312. Van der Waals equation describes fluids composed of particles that are attracted to each other and have a non-negligible volume. Aug 21 2021 XeF4 Molecular Geometry And Bond Angles In order to achieve this the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite 180 degree from each other. What is the volume of 520g of gaseous silicon tetrafluoride SiF4 at STP. For a molecule with the.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The law was discovered by Johannes Diderik van der Waals who later received the Nobel prize for his work on the equation of state for liquids and gases. If you want to cite. Molar mass of CO2 gmol 1201 gmol 2 x 1600 gmol O 4401 gmol CO2 The molar mass of ionic compounds is referred to as the formula mass because ionic. Unlike helium which has a molar mass of about 4 gmol and pitches the voice up SF 6 has a molar mass of about 146 gmol and the speed of sound through the gas is about 134 ms at room temperature pitching the voice down. The arrangement of the molecules in this compound is such that the Carbon atom is in the central atom one Hydrogen atom is on the upper topmost position and the other one is on Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule.
 Source: oneclass.com
Source: oneclass.com
B The number of moles and the mass of oxygen formed by the decomposition of 1252 g of silverI oxide. What is the volume of 520g of gaseous silicon tetrafluoride SiF4 at STP. An example is sulfur hexafluoride SF6 for which writing a Lewis structure with six SF bonds requires that at least 12 electrons be present around the sulfur atom. CH3CCH IN LEWIS DOT DIAGRAMS FNON2H4PCL5CH3OHOH-LiOHNO-2NO2HNO3CH3CHOIF3Asf3SO2-3 H2SO4XeO3 Dot diagrams are almost impossible here in text with hard carriage returns. Reacts with water Solubility.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass of sulfur tetrafluoride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.