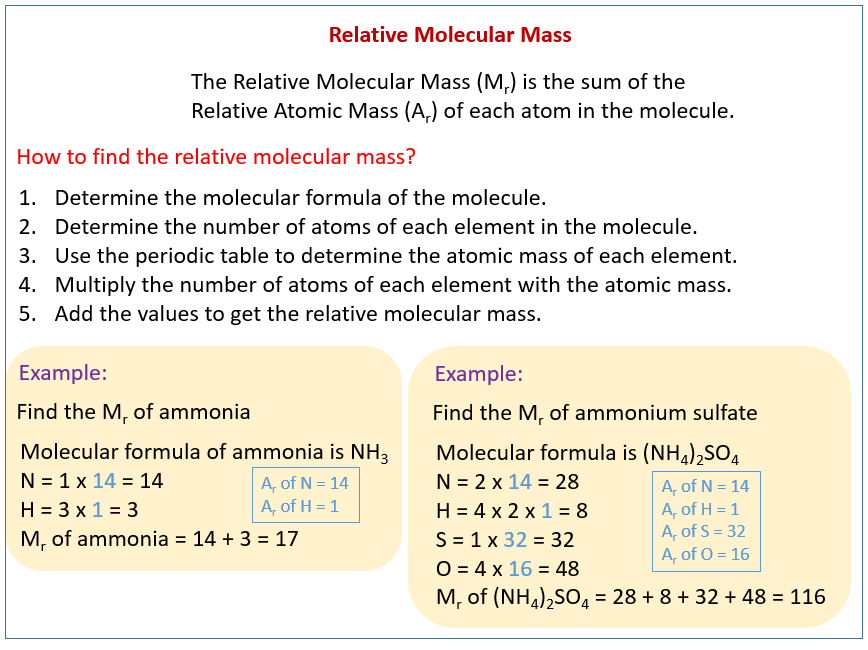
Molar mass of tert butyl alcohol
Molar Mass Of Tert Butyl Alcohol. 80-140 C in the presence of strongly acidic catalysts such as sulfuric or phosphoric acids boron trifluoride activated clays zeolites or strongly acidic ion-exchange resins. 70 yield if tert-butyl phenyl ether is heated to 100 C in. Tert-Butyl alcohol is a colorless solid which melts near room temperature and has a camphor-like odorIt is miscible with water ethanol and diethyl ether. Tert-Butyl alcohol is the simplest tertiary alcohol with a formula of CH 3 3 COH sometimes represented as t-BuOHIt is one of the four isomers of butanol.

Methyl tert-butyl ether 8815 0266 4971 343 0273 Methanol 32042 0564 5126 8097 0224 Ethanol 46069 0645 5139 6148 024 1-Propanol 60096 0622 5368 5175 0254 1-Butanol 74123 0594 5631 4423 026 1-Hexanol 102177 0579 6114 351 0263 2-Propanol 60096 0668 5083 4762 0248 Phenol 94113 0444 6943 613 0243 Ethylene glycol 62068 0487 7197 77 0246 Acetic acid 60053. Tert-Butyl alcohol is a colorless solid which melts near room temperature and has a camphor-like odorIt is miscible with water ethanol and diethyl ether. When dissolved in. Tert-Butyl alcohol is the simplest tertiary alcohol with a formula of CH 3 3 COH sometimes represented as t-BuOHIt is one of the four isomers of butanol. 80-140 C in the presence of strongly acidic catalysts such as sulfuric or phosphoric acids boron trifluoride activated clays zeolites or strongly acidic ion-exchange resins. When tert-butyl chloride is dissolved in water it undergoes a hydrolysis to tert-butyl alcohol.
When tert-butyl chloride is dissolved in water it undergoes a hydrolysis to tert-butyl alcohol.
4-tert-Butylphenol is obtained in ca. Butyl Alcohol C5H10 Cyclopentane C5H10O5 Ribose C5H12 Pentane C5H12O Methyl Tert-butyl Ether C6H10OS2 Allicin C6H12 Cyclohexane C6H12O Cis-3-Hexen-1-ol C6H12O6 Galactose C6H14 Hexane C6H4Cl2 P-Dichlorobenzene C6H5Br Bromobenzene C6H5NO2 Vitamin B3 C6H6 Benzene C6H8O6 Vitamin C C6H8O7 Citric Acid C7H5NO3S Saccharin C7H6O. 70 yield if tert-butyl phenyl ether is heated to 100 C in. We would like to show you a description here but the site wont allow us. 80-140 C in the presence of strongly acidic catalysts such as sulfuric or phosphoric acids boron trifluoride activated clays zeolites or strongly acidic ion-exchange resins. When tert-butyl chloride is dissolved in water it undergoes a hydrolysis to tert-butyl alcohol.
 Source: fishersci.com
Source: fishersci.com
When dissolved in. We would like to show you a description here but the site wont allow us. Methyl tert-butyl ether C5H12O CID 15413 - structure chemical names physical and chemical properties classification patents literature biological activities. 80-140 C in the presence of strongly acidic catalysts such as sulfuric or phosphoric acids boron trifluoride activated clays zeolites or strongly acidic ion-exchange resins. 4-tert-Butylphenol is obtained by reaction of phenol with isobutene at normal or slightly elevated pressure and ca.
 Source: molinstincts.com
Source: molinstincts.com
Methyl tert-butyl ether 8815 0266 4971 343 0273 Methanol 32042 0564 5126 8097 0224 Ethanol 46069 0645 5139 6148 024 1-Propanol 60096 0622 5368 5175 0254 1-Butanol 74123 0594 5631 4423 026 1-Hexanol 102177 0579 6114 351 0263 2-Propanol 60096 0668 5083 4762 0248 Phenol 94113 0444 6943 613 0243 Ethylene glycol 62068 0487 7197 77 0246 Acetic acid 60053. Butyl Alcohol C5H10 Cyclopentane C5H10O5 Ribose C5H12 Pentane C5H12O Methyl Tert-butyl Ether C6H10OS2 Allicin C6H12 Cyclohexane C6H12O Cis-3-Hexen-1-ol C6H12O6 Galactose C6H14 Hexane C6H4Cl2 P-Dichlorobenzene C6H5Br Bromobenzene C6H5NO2 Vitamin B3 C6H6 Benzene C6H8O6 Vitamin C C6H8O7 Citric Acid C7H5NO3S Saccharin C7H6O. Because tert-butanol is a tertiary alcohol the relative stability of the tert-butyl carbocation in the step 2 allows the S N 1 mechanism to be followed whereas a primary alcohol would follow an S N 2 mechanism. When tert-butyl chloride is dissolved in water it undergoes a hydrolysis to tert-butyl alcohol. When dissolved in.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
We would like to show you a description here but the site wont allow us. Methyl tert-butyl ether 8815 0266 4971 343 0273 Methanol 32042 0564 5126 8097 0224 Ethanol 46069 0645 5139 6148 024 1-Propanol 60096 0622 5368 5175 0254 1-Butanol 74123 0594 5631 4423 026 1-Hexanol 102177 0579 6114 351 0263 2-Propanol 60096 0668 5083 4762 0248 Phenol 94113 0444 6943 613 0243 Ethylene glycol 62068 0487 7197 77 0246 Acetic acid 60053. We would like to show you a description here but the site wont allow us. Tert-Butyl alcohol is the simplest tertiary alcohol with a formula of CH 3 3 COH sometimes represented as t-BuOHIt is one of the four isomers of butanol. When tert-butyl chloride is dissolved in water it undergoes a hydrolysis to tert-butyl alcohol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Tert-Butyl alcohol is a colorless solid which melts near room temperature and has a camphor-like odorIt is miscible with water ethanol and diethyl ether. Methyl tert-butyl ether 8815 0266 4971 343 0273 Methanol 32042 0564 5126 8097 0224 Ethanol 46069 0645 5139 6148 024 1-Propanol 60096 0622 5368 5175 0254 1-Butanol 74123 0594 5631 4423 026 1-Hexanol 102177 0579 6114 351 0263 2-Propanol 60096 0668 5083 4762 0248 Phenol 94113 0444 6943 613 0243 Ethylene glycol 62068 0487 7197 77 0246 Acetic acid 60053. 70 yield if tert-butyl phenyl ether is heated to 100 C in. Butyl Alcohol C5H10 Cyclopentane C5H10O5 Ribose C5H12 Pentane C5H12O Methyl Tert-butyl Ether C6H10OS2 Allicin C6H12 Cyclohexane C6H12O Cis-3-Hexen-1-ol C6H12O6 Galactose C6H14 Hexane C6H4Cl2 P-Dichlorobenzene C6H5Br Bromobenzene C6H5NO2 Vitamin B3 C6H6 Benzene C6H8O6 Vitamin C C6H8O7 Citric Acid C7H5NO3S Saccharin C7H6O. When tert-butyl chloride is dissolved in water it undergoes a hydrolysis to tert-butyl alcohol.
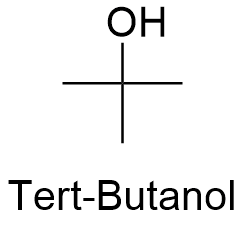 Source: softschools.com
Source: softschools.com
Because tert-butanol is a tertiary alcohol the relative stability of the tert-butyl carbocation in the step 2 allows the S N 1 mechanism to be followed whereas a primary alcohol would follow an S N 2 mechanism. 4-tert-Butylphenol is obtained in ca. 70 yield if tert-butyl phenyl ether is heated to 100 C in. Methyl tert-butyl ether C5H12O CID 15413 - structure chemical names physical and chemical properties classification patents literature biological activities. Butyl Alcohol C5H10 Cyclopentane C5H10O5 Ribose C5H12 Pentane C5H12O Methyl Tert-butyl Ether C6H10OS2 Allicin C6H12 Cyclohexane C6H12O Cis-3-Hexen-1-ol C6H12O6 Galactose C6H14 Hexane C6H4Cl2 P-Dichlorobenzene C6H5Br Bromobenzene C6H5NO2 Vitamin B3 C6H6 Benzene C6H8O6 Vitamin C C6H8O7 Citric Acid C7H5NO3S Saccharin C7H6O.

When tert-butyl chloride is dissolved in water it undergoes a hydrolysis to tert-butyl alcohol. Butyl Alcohol C5H10 Cyclopentane C5H10O5 Ribose C5H12 Pentane C5H12O Methyl Tert-butyl Ether C6H10OS2 Allicin C6H12 Cyclohexane C6H12O Cis-3-Hexen-1-ol C6H12O6 Galactose C6H14 Hexane C6H4Cl2 P-Dichlorobenzene C6H5Br Bromobenzene C6H5NO2 Vitamin B3 C6H6 Benzene C6H8O6 Vitamin C C6H8O7 Citric Acid C7H5NO3S Saccharin C7H6O. Methyl tert-butyl ether 8815 0266 4971 343 0273 Methanol 32042 0564 5126 8097 0224 Ethanol 46069 0645 5139 6148 024 1-Propanol 60096 0622 5368 5175 0254 1-Butanol 74123 0594 5631 4423 026 1-Hexanol 102177 0579 6114 351 0263 2-Propanol 60096 0668 5083 4762 0248 Phenol 94113 0444 6943 613 0243 Ethylene glycol 62068 0487 7197 77 0246 Acetic acid 60053. Because tert-butanol is a tertiary alcohol the relative stability of the tert-butyl carbocation in the step 2 allows the S N 1 mechanism to be followed whereas a primary alcohol would follow an S N 2 mechanism. Tert-Butyl alcohol is the simplest tertiary alcohol with a formula of CH 3 3 COH sometimes represented as t-BuOHIt is one of the four isomers of butanol.
 Source: tcichemicals.com
Source: tcichemicals.com
Methyl tert-butyl ether C5H12O CID 15413 - structure chemical names physical and chemical properties classification patents literature biological activities. When tert-butyl chloride is dissolved in water it undergoes a hydrolysis to tert-butyl alcohol. Methyl tert-butyl ether 8815 0266 4971 343 0273 Methanol 32042 0564 5126 8097 0224 Ethanol 46069 0645 5139 6148 024 1-Propanol 60096 0622 5368 5175 0254 1-Butanol 74123 0594 5631 4423 026 1-Hexanol 102177 0579 6114 351 0263 2-Propanol 60096 0668 5083 4762 0248 Phenol 94113 0444 6943 613 0243 Ethylene glycol 62068 0487 7197 77 0246 Acetic acid 60053. Methyl tert-butyl ether C5H12O CID 15413 - structure chemical names physical and chemical properties classification patents literature biological activities. Tert-Butyl alcohol is the simplest tertiary alcohol with a formula of CH 3 3 COH sometimes represented as t-BuOHIt is one of the four isomers of butanol.

Butyl Alcohol C5H10 Cyclopentane C5H10O5 Ribose C5H12 Pentane C5H12O Methyl Tert-butyl Ether C6H10OS2 Allicin C6H12 Cyclohexane C6H12O Cis-3-Hexen-1-ol C6H12O6 Galactose C6H14 Hexane C6H4Cl2 P-Dichlorobenzene C6H5Br Bromobenzene C6H5NO2 Vitamin B3 C6H6 Benzene C6H8O6 Vitamin C C6H8O7 Citric Acid C7H5NO3S Saccharin C7H6O. When dissolved in. We would like to show you a description here but the site wont allow us. Tert-Butyl alcohol is a colorless solid which melts near room temperature and has a camphor-like odorIt is miscible with water ethanol and diethyl ether. 80-140 C in the presence of strongly acidic catalysts such as sulfuric or phosphoric acids boron trifluoride activated clays zeolites or strongly acidic ion-exchange resins.
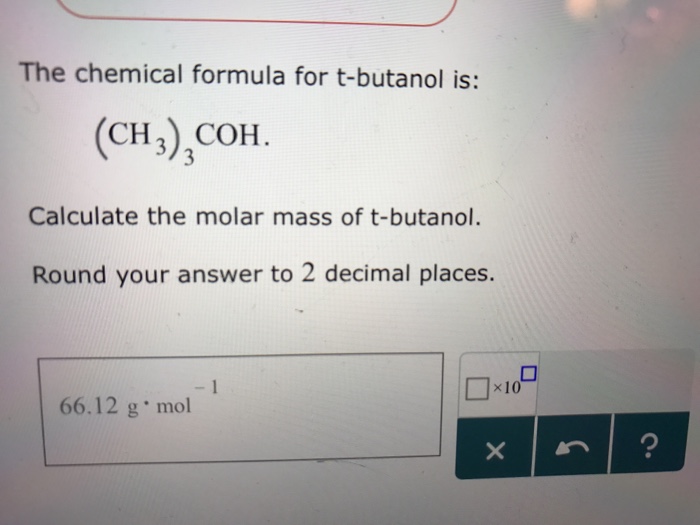
4-tert-Butylphenol is obtained in ca. Tert-Butyl alcohol is a colorless solid which melts near room temperature and has a camphor-like odorIt is miscible with water ethanol and diethyl ether. 80-140 C in the presence of strongly acidic catalysts such as sulfuric or phosphoric acids boron trifluoride activated clays zeolites or strongly acidic ion-exchange resins. 4-tert-Butylphenol is obtained in ca. Butyl Alcohol C5H10 Cyclopentane C5H10O5 Ribose C5H12 Pentane C5H12O Methyl Tert-butyl Ether C6H10OS2 Allicin C6H12 Cyclohexane C6H12O Cis-3-Hexen-1-ol C6H12O6 Galactose C6H14 Hexane C6H4Cl2 P-Dichlorobenzene C6H5Br Bromobenzene C6H5NO2 Vitamin B3 C6H6 Benzene C6H8O6 Vitamin C C6H8O7 Citric Acid C7H5NO3S Saccharin C7H6O.

We would like to show you a description here but the site wont allow us. 80-140 C in the presence of strongly acidic catalysts such as sulfuric or phosphoric acids boron trifluoride activated clays zeolites or strongly acidic ion-exchange resins. We would like to show you a description here but the site wont allow us. 70 yield if tert-butyl phenyl ether is heated to 100 C in. Methyl tert-butyl ether C5H12O CID 15413 - structure chemical names physical and chemical properties classification patents literature biological activities.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass of tert butyl alcohol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.