Molar mass oh naoh
Molar Mass Oh Naoh. 1 Determine mass of 100 L of solution. It has a density of 108 gmL. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. Expatica is the international communitys online home away from home.
 Naoh Molar Mass About Substance In Simple Words From studybay.com
Naoh Molar Mass About Substance In Simple Words From studybay.com
To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. 108 gmL 1000 mL 1080 g. NaOH Na aq. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. How does buffering a solution change the solutions behavior. Commercial bleach solution contains 525 by mass of NaClO in water.
Caculate the molarity of this solution.
108 gmL 1000 mL 1080 g. Calculate the molar ratios. Commercial bleach solution contains 525 by mass of NaClO in water. How does buffering a solution change the solutions behavior. A must-read for English-speaking expatriates and internationals across Europe Expatica provides a tailored local news service and essential information on living working and moving to your country of choice. How does Benedicts solution change colour.

Molar mass of NaClO 744 gmol Solution. NaOH aq moles volume L 375 10-3 mol 0025 L 015 mol L-1. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. With in-depth features Expatica brings the international community closer together. Commercial bleach solution contains 525 by mass of NaClO in water.
 Source: study.com
Source: study.com
How does Benedicts solution change colour. Caculate the molarity of this solution. This gives you a molar ratio of Al to I_2 of 0044480009456 Usually you divide each number in the fraction by the smaller number of moles. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. Calculate the molar ratios.
 Source: youtube.com
Source: youtube.com
NaOH is a strong base so it fully dissociates in water. Calculate the concentration of OH-due to NaOH once NaOH is added to the acid. Calculate the molar ratios. This gives you a molar ratio of Al to I_2 of 0044480009456 Usually you divide each number in the fraction by the smaller number of moles. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.
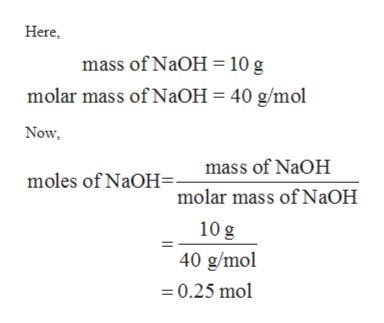 Source: bartleby.com
Source: bartleby.com
Caculate the molarity of this solution. Calculate theoretical concentration of NaOH when the NaOH is added to the acid assuming no reaction occurs. NaOH Na aq. How does buffering a solution change the solutions behavior. With in-depth features Expatica brings the international community closer together.
 Source: studybay.com
Source: studybay.com
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Caculate the molarity of this solution. Expatica is the international communitys online home away from home. Calculate the concentration of OH-due to NaOH once NaOH is added to the acid. How does buffering a solution change the solutions behavior.
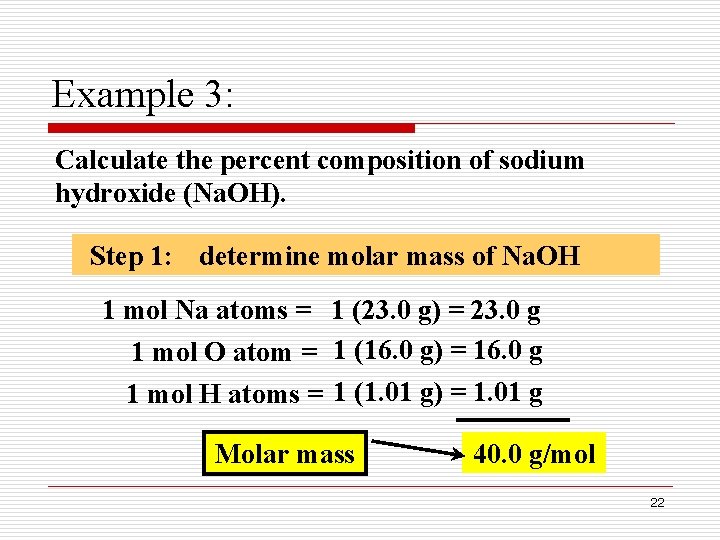 Source: slidetodoc.com
Source: slidetodoc.com
To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. NaOH is a strong base so it fully dissociates in water. Caculate the molarity of this solution. Calculate the amount of 1 M NaOH aqueous solution needed to make 100 mL of 05 M NaOH aqueous solution. NaOH aq moles volume L 375 10-3 mol 0025 L 015 mol L-1.
This gives a ratio in which no number is less than 1. Calculate the molar ratios. Calculate the amount of 1 M NaOH aqueous solution needed to make 100 mL of 05 M NaOH aqueous solution. Molar Mass of Frequently Calculated Chemicals. NaOH Na aq.
 Source: topperlearning.com
Source: topperlearning.com
This gives a ratio in which no number is less than 1. Expatica is the international communitys online home away from home. It has a density of 108 gmL. Caculate the molarity of this solution. Assume you have 100 L of solution.
 Source: youtube.com
Source: youtube.com
This gives you a molar ratio of Al to I_2 of 0044480009456 Usually you divide each number in the fraction by the smaller number of moles. A must-read for English-speaking expatriates and internationals across Europe Expatica provides a tailored local news service and essential information on living working and moving to your country of choice. It has a density of 108 gmL. Calculate the amount of 1 M NaOH aqueous solution needed to make 100 mL of 05 M NaOH aqueous solution. How does Benedicts solution change colour.
 Source: youtube.com
Source: youtube.com
Calculate the amount of 1 M NaOH aqueous solution needed to make 100 mL of 05 M NaOH aqueous solution. Calculate the molar ratios. This gives you a molar ratio of Al to I_2 of 0044480009456 Usually you divide each number in the fraction by the smaller number of moles. 108 gmL 1000 mL 1080 g. Commercial bleach solution contains 525 by mass of NaClO in water.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass oh naoh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






