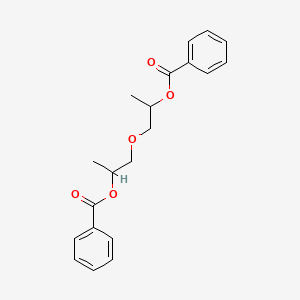
Molar mass sodium formate
Molar Mass Sodium Formate. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Esters of the low molar mass alkanoic acids and alkanols have fragrant. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Molar mass of naf email protected.
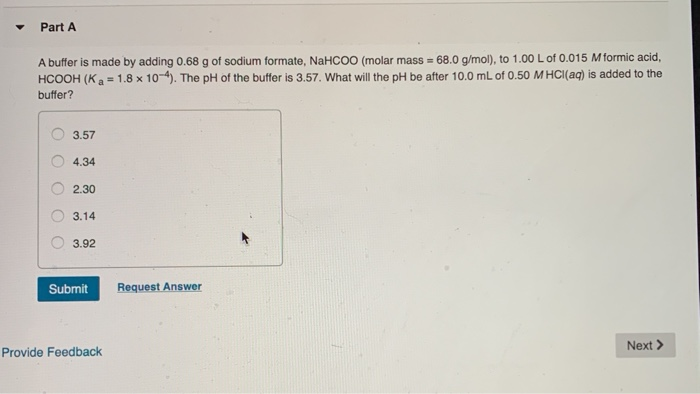 Solved Part A A Buffer Is Made By Adding 0 68 G Of Sodium Chegg Com From chegg.com
Solved Part A A Buffer Is Made By Adding 0 68 G Of Sodium Chegg Com From chegg.com
Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. Buffers work best if the solutions pH is close to the buffers pK 1. If you want to compute the pH of a given sodium phosphate buffer you just need to enter the pKa into the calculator that best suits the pH you want to calculate. Formaldehyde reacts with a base like sodium hydroxide forms sodium formate and methanol. Sodium hydrogen tartrate. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand.
It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite.
526 K Boiling point. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. 6HCHO 4NH 3 CH 26N 4 6H 2 O. The buffer pH. The chemical equation is given below.
 Source: youtube.com
Source: youtube.com
It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. Sodium hydrogen tartrate. The chemical equation is given below. Formaldehyde reacts with ammonia forms formamidine and water. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
Source: socratic.org
If you want to compute the pH of a given sodium phosphate buffer you just need to enter the pKa into the calculator that best suits the pH you want to calculate. Esters of the low molar mass alkanoic acids and alkanols have fragrant. 68007 gmol Appearance white granules deliquescent. If you want to compute the pH of a given sodium phosphate buffer you just need to enter the pKa into the calculator that best suits the pH you want to calculate. An aqueous solution of sodium carbonate or alternatively sodium hydrogen carbonate 8 is used to neutralise any remaining acid.
 Source: radsshbchemicals.biz
Source: radsshbchemicals.biz
Methyl formate is a formate ester resulting from the formal condensation of formic acid with methanol. Formaldehyde reacts with a base like sodium hydroxide forms sodium formate and methanol. Thermochemistry Heat capacity C 827 Jmol K Std molar entropy S o. Esters of the low molar mass alkanoic acids and alkanols have fragrant. Insoluble in ether soluble in glycerol alcohol formic acid.
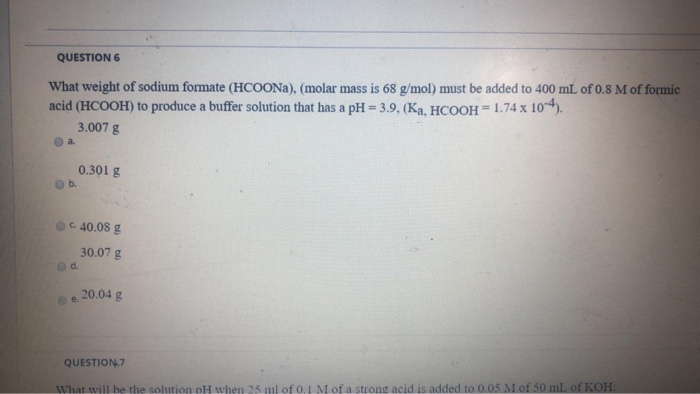 Source: chegg.com
Source: chegg.com
526 K Boiling point. CO 3 2-aq 2H aq CO 2 g H 2 Ol As sodium carbonate is added bubbles of carbon. How to calculate the pH of a buffer solution. A low-boiling 315 colourless flammable liquid it has been used as a fumigant and larvicide for tobacco and food crops. Uses of Formaldehyde CH 2 O.
 Source: chegg.com
Source: chegg.com
CO 3 2-aq 2H aq CO 2 g H 2 Ol As sodium carbonate is added bubbles of carbon. 526 K Boiling point. Decomposes Solubility in water. 6HCHO 4NH 3 CH 26N 4 6H 2 O. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming.
 Source: en.wikipedia.org
Source: en.wikipedia.org
526 K Boiling point. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. 2HCHO NaOH HCOONa CH 3 OH. CO 3 2-aq 2H aq CO 2 g H 2 Ol As sodium carbonate is added bubbles of carbon. Esters of the low molar mass alkanoic acids and alkanols have fragrant.
Source: chemspider.com
Formaldehyde reacts with ammonia forms formamidine and water. A formation rate of formate of 1449 μmol h 1 cm 2 with a formate FE of 93 could be achieved at 098 V versus RHE significantly better than those reported to date Supplementary Table 1. NaHC 4 H 4 O 6. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. The buffer pH.
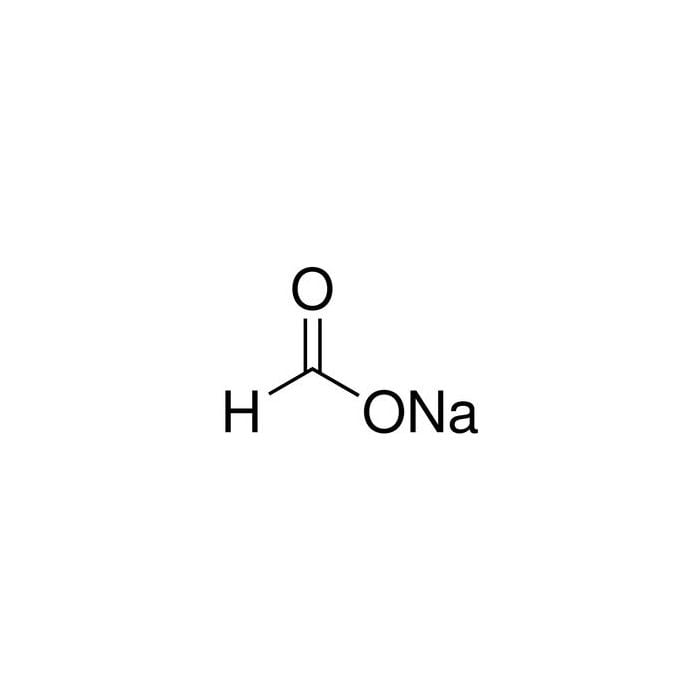 Source: lab.honeywell.com
Source: lab.honeywell.com
Na 2 HPO 4. 6HCHO 4NH 3 CH 26N 4 6H 2 O. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Methyl formate is a formate ester resulting from the formal condensation of formic acid with methanol. Molar mass of naf email protected.
 Source: numerade.com
Source: numerade.com
The chemical equation is given below. NaHC 4 H 4 O 6. 68007 gmol Appearance white granules deliquescent. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming. Na 2 HPO 4.
 Source: degruyter.com
Source: degruyter.com
A formation rate of formate of 1449 μmol h 1 cm 2 with a formate FE of 93 could be achieved at 098 V versus RHE significantly better than those reported to date Supplementary Table 1. 68007 gmol Appearance white granules deliquescent. A formation rate of formate of 1449 μmol h 1 cm 2 with a formate FE of 93 could be achieved at 098 V versus RHE significantly better than those reported to date Supplementary Table 1. Methyl formate is a formate ester resulting from the formal condensation of formic acid with methanol. Formaldehyde reacts with a base like sodium hydroxide forms sodium formate and methanol.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass sodium formate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.