Molecular mass of carbon tetrachloride
Molecular Mass Of Carbon Tetrachloride. The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. The molar mass of carbon tetrachloride is 15381 grams per mole. Titanium tetrachloride is the inorganic compound with the formula TiCl 4. It has a colourless appearance and a sweet odour.
 Molar Mass Of Ccl4 Carbon Tetrachloride Youtube From youtube.com
Molar Mass Of Ccl4 Carbon Tetrachloride Youtube From youtube.com
The molar mass of carbon tetrachloride is 15381 grams per mole. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. The melting point of carbon tetrachloride is -2293 o C whereas the boiling point of this compound corresponds. Under standard conditions this compound exists in the liquid state. It is sometimes referred to as tickle or tickle 4 due to the. Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chloride.
The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre.
Titanium tetrachloride is the inorganic compound with the formula TiCl 4. The molar mass of carbon tetrachloride is 15381 grams per mole. Under standard conditions this compound exists in the liquid state. TiCl 4 is a volatile liquid. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre.
 Source: wiredfaculty.com
Source: wiredfaculty.com
The molar mass of carbon tetrachloride is 15381 grams per mole. The melting point of carbon tetrachloride is -2293 o C whereas the boiling point of this compound corresponds. Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chloride. It is sometimes referred to as tickle or tickle 4 due to the. The molar mass of carbon tetrachloride is 15381 grams per mole.
 Source: brainly.in
Source: brainly.in
The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chloride. The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. The molar mass of carbon tetrachloride is 15381 grams per mole. It has a colourless appearance and a sweet odour.
 Source: slideplayer.com
Source: slideplayer.com
Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chloride. It is sometimes referred to as tickle or tickle 4 due to the. TiCl 4 is a volatile liquid. Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chloride. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide.
 Source: toppr.com
Source: toppr.com
The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. Under standard conditions this compound exists in the liquid state. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. The melting point of carbon tetrachloride is -2293 o C whereas the boiling point of this compound corresponds.
 Source: slideplayer.com
Source: slideplayer.com
The molar mass of carbon tetrachloride is 15381 grams per mole. TiCl 4 is a volatile liquid. It is sometimes referred to as tickle or tickle 4 due to the. The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. Titanium tetrachloride is the inorganic compound with the formula TiCl 4.
 Source: slideplayer.com
Source: slideplayer.com
Titanium tetrachloride is the inorganic compound with the formula TiCl 4. The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. The molar mass of carbon tetrachloride is 15381 grams per mole. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. Under standard conditions this compound exists in the liquid state.
 Source: youtube.com
Source: youtube.com
TiCl 4 is a volatile liquid. It is sometimes referred to as tickle or tickle 4 due to the. The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. Under standard conditions this compound exists in the liquid state. It has a colourless appearance and a sweet odour.
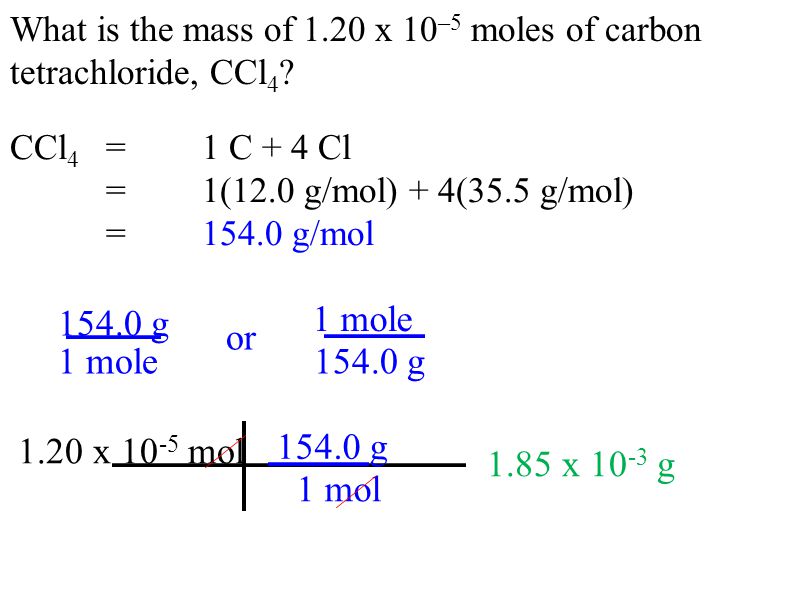 Source: slideplayer.com
Source: slideplayer.com
Under standard conditions this compound exists in the liquid state. Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chloride. The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. It has a colourless appearance and a sweet odour. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide.
 Source: youtube.com
Source: youtube.com
Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chloride. It has a colourless appearance and a sweet odour. TiCl 4 is a volatile liquid. Titanium tetrachloride is the inorganic compound with the formula TiCl 4. The melting point of carbon tetrachloride is -2293 o C whereas the boiling point of this compound corresponds.
 Source: youtube.com
Source: youtube.com
TiCl 4 is a volatile liquid. Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chloride. The density of this compound in its liquid state corresponds to 15867 grams per cubic centimetre. TiCl 4 is a volatile liquid. It has a colourless appearance and a sweet odour.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molecular mass of carbon tetrachloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.