Molecular mass of dinitrogen monoxide
Molecular Mass Of Dinitrogen Monoxide. For example the molecular weight of water would be obtained by the following process. 60008 gmol 1 Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. C12H22O11 mass - 1201 x 12 1008 x 22 16 x 11 3423 gmol - 124g C12H22O113423gmol C12H22O11 00326 mol C12H22O11 3. Use avogadros number to get rid of moles.
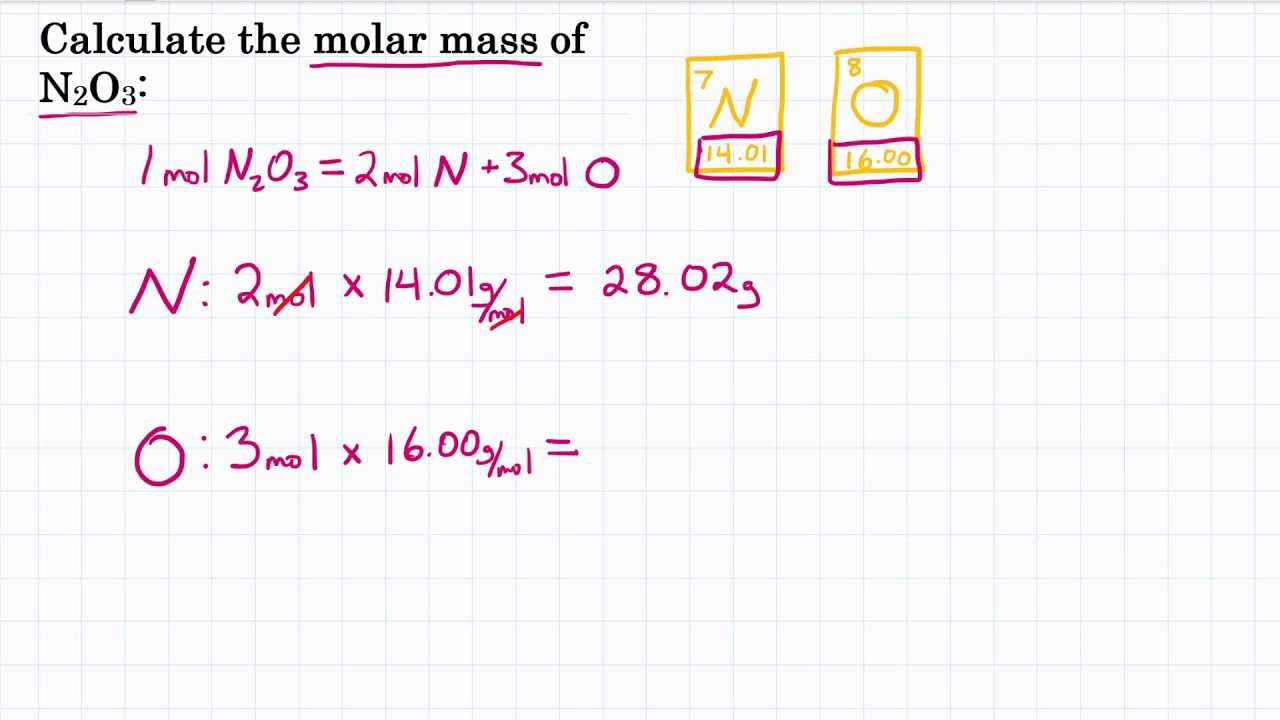 Calculate The Molar Mass Of N2o3 Dinitrogen Trioxide Molar Mass Practice Youtube From youtube.com
Calculate The Molar Mass Of N2o3 Dinitrogen Trioxide Molar Mass Practice Youtube From youtube.com
Molecular mass of H 2 O 2 x atomic mass of H 1 x atomic mass of O 2 x 100797 1 x 159994 amu 1802 amu. Chemical formula for quartz. Number of O atoms 5621022 find molar mass divide 280 by molar mass. Fe 2 O 3. 60008 gmol 1 Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm.
It oxidizes sulfur dichloride to thionyl chloride.
What is the name for the compound NO2. Since it dissolves completely forming Na and Cl- ions it is classified as a strong electrolyte. C 2 H 6 O 2. 252 kg to g - 252 x 1000 25200 - mass C2H2 - 1201 x 2 1008 x 2 26036 gmol C2H2. 2 SO 3 N 2 O 5 NO 2 2 S 2 O 7 Oxidant. What is the formula weight of MgNO32.
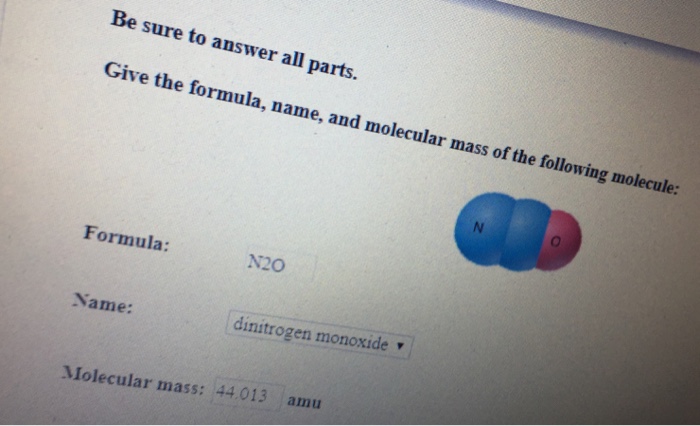
Number of O atoms 5621022 find molar mass divide 280 by molar mass. C 2 H 5 OH. It oxidizes sulfur dichloride to thionyl chloride. What is the formula weight of MgNO32. The molecularformula mass is numerically equal to the mass of one mole of the substance.
 Source: slideserve.com
Source: slideserve.com
With pyridine it give the sulfur trioxide. 926 kJ mol H This is the method for the manufacture of ammonia by the Haber process. 673K200atm 22 Fe 3 1 3H g N g 2NH g. What is the name for the compound NO2. Sulfur trioxide is an oxidant.
 Source: clutchprep.com
Source: clutchprep.com
N 2 O 5. Carbon trioxide CO 3 is an unstable oxide of carbon an oxocarbon. C12H22O11 mass - 1201 x 12 1008 x 22 16 x 11 3423 gmol - 124g C12H22O113423gmol C12H22O11 00326 mol C12H22O11 3. 252 kg C2H2 4. With dinitrogen it forms ammonia.
 Source: slideplayer.com
Source: slideplayer.com
Fe 2 O 3. Chemical formula for quartz. 926 kJ mol H This is the method for the manufacture of ammonia by the Haber process. For example the molecular weight of water would be obtained by the following process. Molecular mass of H 2 O 2 x atomic mass of H 1 x atomic mass of O 2 x 100797 1 x 159994 amu 1802 amu.
 Source: youtube.com
Source: youtube.com
60008 gmol 1 Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. The possible isomers of carbon trioxide include ones with molecular symmetry point groups C s D 3h and C 2v. 2 SO 3 N 2 O 5 NO 2 2 S 2 O 7 Oxidant. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. Density of a metal d.
 Source: youtube.com
Source: youtube.com
Carbon trioxide CO 3 is an unstable oxide of carbon an oxocarbon. Since it dissolves completely forming Na and Cl- ions it is classified as a strong electrolyte. 60008 gmol 1 Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. What is the name for the compound NO2. For example the molecular weight of water would be obtained by the following process.
 Source: clutchprep.com
Source: clutchprep.com
Chemical formula for quartz. With many metals it combines at a high temperature to yield the corresponding hydrides section 95. N 2 O 5. Density of a metal d. What is the name for the compound NO2.
 Source: youtube.com
Source: youtube.com
CoCl2 binary ionic d. Q-The mass of an electron is 9. Number of O atoms 5621022 find molar mass divide 280 by molar mass. H 2 SO 4 aq Ba OH 2 aq — BaSO 4 s 2H 2 O l So the molecular form of the equation is shown above. N 2 O 5.
 Source: youtube.com
Source: youtube.com
C 2 H 6 O 2. The molecularformula mass is numerically equal to the mass of one mole of the substance. N 2 O 5. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. Density of a metal d.
 Source: chegg.com
Source: chegg.com
CCl4 mass - 1201 3545 x 4 15381 gmol - 725g CCl415381gmol CCl4 0471 mol CCl4 2. 252 kg to g - 252 x 1000 25200 - mass C2H2 - 1201 x 2 1008 x 2 26036 gmol C2H2. SO 3 SCl 2 SOCl 2 SO 2 Lewis acid. For example the molecular weight of water would be obtained by the following process. The possible isomers of carbon trioxide include ones with molecular symmetry point groups C s D 3h and C 2v.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molecular mass of dinitrogen monoxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.