Molecular mass of naclo
Molecular Mass Of Naclo. These salts formed when chlorine reacts with alkaline metals hydroxide. In addition to high Li conductivity 15 103 siemens per centimetre at room temperature along the molecular chain direction the Cu2-coordinated cellulose ion conductor also exhibits a. Where M 0 is the molecular weight or molar mass of the monomer or repeat unit. Image will be uploaded soon.

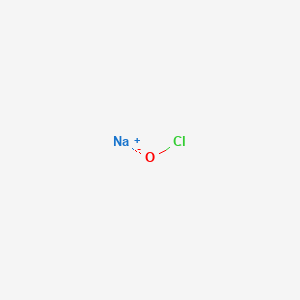
The empirical formula of a chemical compound represents the ratio of the elements present in that compound. It is also known as the sodium salt of hypochlorous acid HOCl. Used to produce disinfectants. Structure of Hydroxide OH Structure of Hydroxide. Li 2 Oaq CO 2 g Li 2 CO 3 aq What mass of Fe 2 O 3 must be reacted to generate 324 g. Other general names.
It is also known as the sodium salt of hypochlorous acid HOCl.
When the temperature reaches a certain level there is a phase change. The relations among amount of substance in. Used as food preservatives to prevent bacteria and mold growing in food. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. Cl 2 2 NaOH NaCl NaClO H 2 O. Used to produce disinfectants.
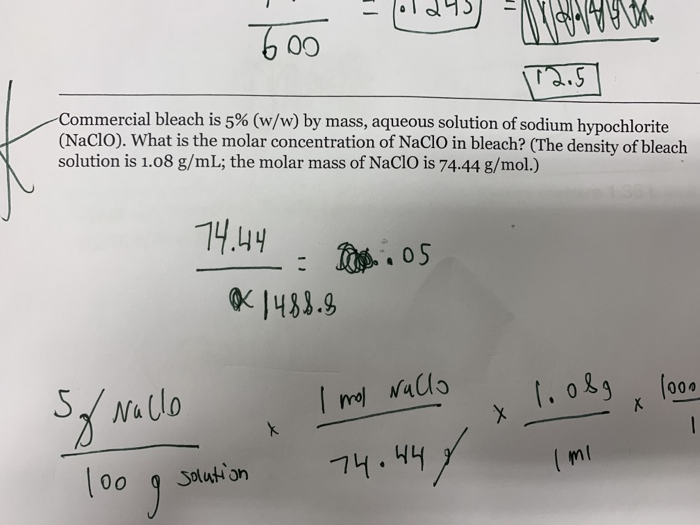 Source: chegg.com
Source: chegg.com
In this tutorial we will discuss HypochloriteClO- lewis structure molecular geometry polarity hybridization etc. It is also known as the sodium salt of hypochlorous acid HOCl. Sodium hypochlorite is a salt made up of sodium cation and hypochlorite anion. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. In molecular formulae the elements are denoted by their respective symbols as in the periodic table and the number of atoms of each element in the molecule is written in subscript.
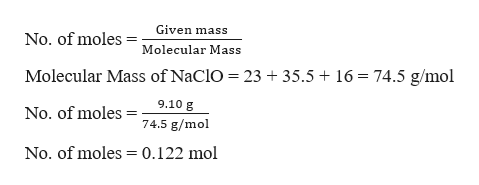 Source: bartleby.com
Source: bartleby.com
A chlorite compound is a compound that contains this group with chlorine in the oxidation state of 3. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming. What mass of O 2 can be generated by the decomposition of 1000 g of NaClO 3. A chlorite compound is a compound that contains this group with chlorine in the oxidation state of 3. For example- the molecular formula for glucose is C 6 H 12 O 6.
 Source: slideplayer.com
Source: slideplayer.com
Where M 0 is the molecular weight or molar mass of the monomer or repeat unit. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. A chlorite compound is a compound that contains this group with chlorine in the oxidation state of 3. Antiformin bleach chloride of soda. The relation between molecular formula mass and molar mass.
Uses of Hydroxide OH Hydroxide is used to produce fuel cells. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming. These salts formed when chlorine reacts with alkaline metals hydroxide. Molecular Weight of Hydroxide. The relations among amount of substance in.
 Source: slideplayer.com
Source: slideplayer.com
This means that the molecular compound changes from solid to liquid or from liquid to gas. Cl 2 2 NaOH NaCl NaClO H 2 O. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. By definition the molar mass of the end groups should be included in the molecular weight of a polymer but the corresponding quantity is not included in the degree of polymerization. It is also known as the sodium salt of hypochlorous acid HOCl.
 Source: study.com
Source: study.com
It is also known as the sodium salt of hypochlorous acid HOCl. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming. For example- the molecular formula for glucose is C 6 H 12 O 6. These salts formed when chlorine reacts with alkaline metals hydroxide. Cl 2 2 NaOH NaCl NaClO H 2 O.

These salts formed when chlorine reacts with alkaline metals hydroxide. Other general names. Hypochlorite salts are used as the bleaching agents disinfection and for water treatment. Molecular compounds do not have a definite melting point and boiling point. The empirical formula of a chemical compound represents the ratio of the elements present in that compound.
 Source: tcichemicals.com
Source: tcichemicals.com
Hypochlorite salts are used as the bleaching agents disinfection and for water treatment. Sodium hypochlorite is a salt made up of sodium cation and hypochlorite anion. NaOCl or NaClO. Used to produce disinfectants. Other general names.
 Source: slideplayer.com
Source: slideplayer.com
In this tutorial we will discuss HypochloriteClO- lewis structure molecular geometry polarity hybridization etc. When the temperature reaches a certain level there is a phase change. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming. The relation between molecular formula mass and molar mass. Structure of Hydroxide OH Structure of Hydroxide.
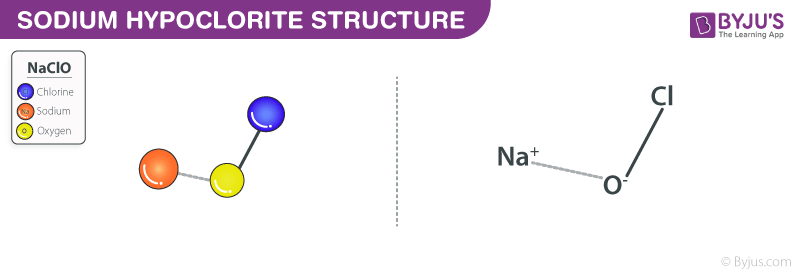 Source: byjus.com
Source: byjus.com
In this book in accord with common usage we use the term molecular weight rather than molar mass when referring to polymers. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming. Used as food preservatives to prevent bacteria and mold growing in food. Hypochlorite salts are used as the bleaching agents disinfection and for water treatment. Used to produce disinfectants.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molecular mass of naclo by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.