Molecular mass of phosphorus oxide
Molecular Mass Of Phosphorus Oxide. It is a phosphorus. Corrosive to metals and tissue and moderately toxic. An oxide of nitrogen has 696 by mass of oxygen. Molar mass has units of grams per mole gmol.
 Phosphorus Pentoxide Wikipedia From en.wikipedia.org
Phosphorus Pentoxide Wikipedia From en.wikipedia.org
What is the empirical formula of this compound of copper. Diphosphonate2- is a divalent inorganic anion obtained by removal of both protons from diphosphonic acid. PhosphorusIII oxide P 4 O 6 also called tetraphosphorus hexoxide is the anhydride of POH 3 the minor tautomer of phosphorous acid. In rocks it is combined with metals and nonmetals in the form of oxides that are acidic such as those of sulfur carbon aluminum and phosphorus or basic such as those of. Nitric oxide is also a heteronuclear diatomic molecule a class of molecules whose study spawned early modern theories. The relations among amount of substance in.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
An oxide of nitrogen has 696 by mass of oxygen. What is the empirical formula of this compound of copper. Calculate the mass percent of carbon nitrogen and oxygen in acetamide C 2 H 5 NO. What is its molecular formula. Cu 259 S 127 O 574 H 4. The relations among amount of substance in.
 Source: softschools.com
Source: softschools.com
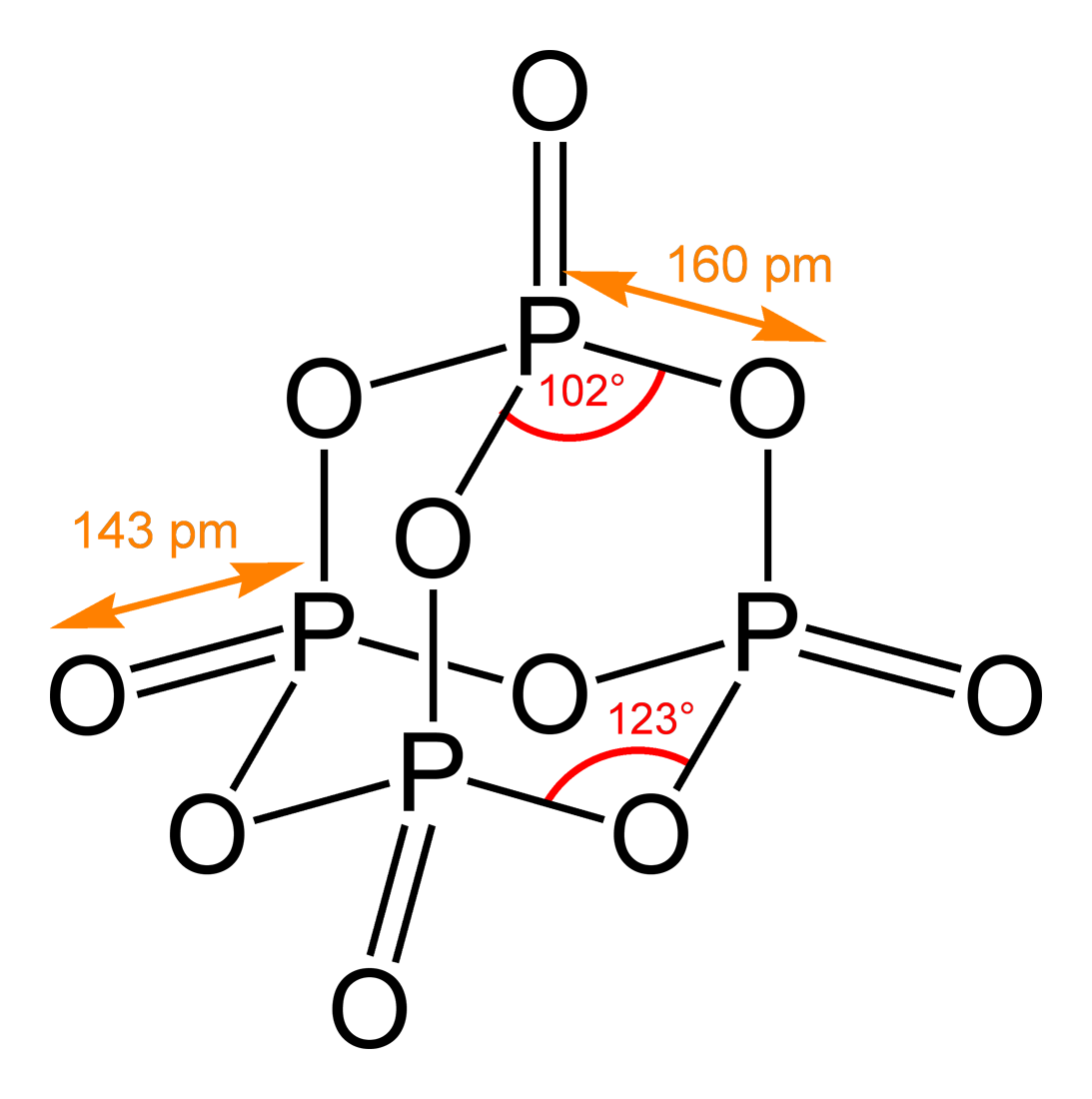
What is the empirical formula of this compound of copper. The relation between molecular formula mass and molar mass. A 5051 g sample of a compound made from phosphorus and chlorine is decomposed. The structure of P 4 O 6 is like that of P 4 O 10 without the terminal oxide groups. What is the empirical formula of this compound of copper.

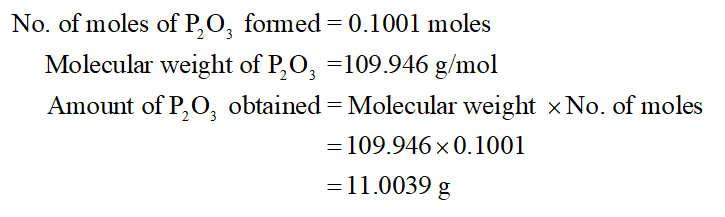
5 The molar mass M of a substance is the mass of one mole of entities atoms molecules or formula units of the substance. Analysis of the products showed that 1139 g of phosphorus atoms were produced. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. What is the empirical formula of this compound of copper. PhosphorusI and phosphorusII A stable diphosphene a derivative of.
 Source: youtube.com
Source: youtube.com
Phosphoric anhydride appears as a white amorphous powder. Analysis of the products showed that 1139 g of phosphorus atoms were produced. It is a phosphorus. 4 To obtain one mole of copper atoms 602 x 10 23 atoms weigh out 6355 g copper. Diphosphonate2- is a divalent inorganic anion obtained by removal of both protons from diphosphonic acid.
 Source: bartleby.com
Source: bartleby.com
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. In rocks it is combined with metals and nonmetals in the form of oxides that are acidic such as those of sulfur carbon aluminum and phosphorus or basic such as those of. An oxide of nitrogen has 696 by mass of oxygen. Corrosive to metals and tissue and moderately toxic. When 25000 g of an oxide of mercury Hg x O y is.
 Source: youtube.com
Source: youtube.com
PhosphorusI and phosphorusII A stable diphosphene a derivative of. What is the empirical formula. It has the following percentages by mass. Molar mass has units of grams per mole gmol. 4 To obtain one mole of copper atoms 602 x 10 23 atoms weigh out 6355 g copper.
 Source: slideplayer.com
Source: slideplayer.com
The relation between molecular formula mass and molar mass. Corrosive to metals and tissue and moderately toxic. Molar mass has units of grams per mole gmol. What is the empirical formula of the compound. Analysis of the products showed that 1139 g of phosphorus atoms were produced.
 Source: toppr.com
Source: toppr.com
What is the empirical formula of the compound. It is a phosphorus. 4 To obtain one mole of copper atoms 602 x 10 23 atoms weigh out 6355 g copper. Analysis of the products showed that 1139 g of phosphorus atoms were produced. The relations among amount of substance in.
 Source: en.wikipedia.org
Source: en.wikipedia.org
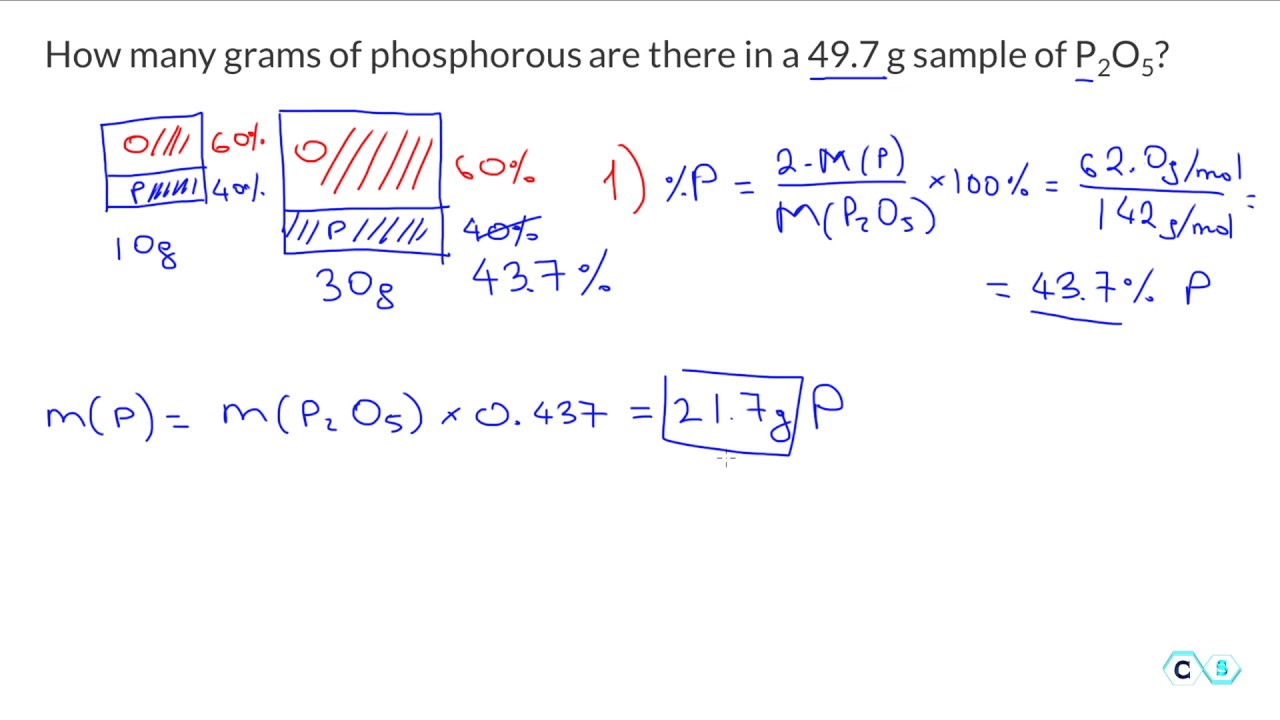
Molar mass has units of grams per mole gmol. What is the empirical formula of the phosphorus oxide that has 437 by mass of phosphorus and 284 by mass oxygen. Diphosphonate2- is a divalent inorganic anion obtained by removal of both protons from diphosphonic acid. 5 The molar mass M of a substance is the mass of one mole of entities atoms molecules or formula units of the substance. 4 To obtain one mole of copper atoms 602 x 10 23 atoms weigh out 6355 g copper.
 Source: toppr.com
Source: toppr.com
When 25000 g of an oxide of mercury Hg x O y is. Analysis of the products showed that 1139 g of phosphorus atoms were produced. Phosphoric anhydride appears as a white amorphous powder. The structure of P 4 O 6 is like that of P 4 O 10 without the terminal oxide groups. When 25000 g of an oxide of mercury Hg x O y is.
 Source: eurekaelearning.com
Source: eurekaelearning.com
Diphosphonate2- is a divalent inorganic anion obtained by removal of both protons from diphosphonic acid. Molar mass has units of grams per mole gmol. Calculate the mass percent of carbon nitrogen and oxygen in acetamide C 2 H 5 NO. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. What is the empirical formula of the compound.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molecular mass of phosphorus oxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






