Molecular weight of nitrogen monoxide
Molecular Weight Of Nitrogen Monoxide. Key Takeaways Key Points. In ambient air the oxides of. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Read on to know more Read on to know more We all know that a compound is formed by combining two or more elements metal or.
Nitrogen - Density and Specific Weight vs. Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight or molar mass can be calculated by adding the weight of each component. Its molecular weight is 4601 gmol melting point 112 C boiling point 2115 C and density 159 air 1. The main neutral hydride of nitrogen is ammonia NH 3 which has a pK b of 92 and is thus a weak. S M M air where Sgas specific gravity Mgas molecular weight M air 2896443 gmole molecular weight of standard air - CRC 1983. Molecular diffusion often simply called diffusion is the thermal motion of all liquid or gas particles at temperatures above absolute zeroThe rate of this movement is a function of temperature viscosity of the fluid and the size mass of the particles.
Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia carbon monoxide hydrogen hydrogen sulfide or methane.
The high molecular weight makes it a very tough material but results in less efficient packing of the chains into the crystal structure as evidenced by densities of less than high-density polyethylene for example 09300935 gcm 3. Nitrogen - Density and Specific Weight vs. Carbon monoxide - CO - 0 to trace ppm Ammonia. Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight or molar mass can be calculated by adding the weight of each component. Levels of 15 03 ppm 3 06 SD mgm 3 nitrogen dioxide and 114 21 ppm nitrogen oxides were found in whole exhaust and 12 026 ppm 24 05 mgm 3 nitrogen dioxide and 99 18 ppm nitrogen oxides in filtered exhaust. S M M air where Sgas specific gravity Mgas molecular weight M air 2896443 gmole molecular weight of standard air - CRC 1983.
 Source: webelements.com
Source: webelements.com
S M M air where Sgas specific gravity Mgas molecular weight M air 2896443 gmole molecular weight of standard air - CRC 1983. National Fire Protection Association. Air - Prandtl Number - Prandtl number for air vs. Levels of 15 03 ppm 3 06 SD mgm 3 nitrogen dioxide and 114 21 ppm nitrogen oxides were found in whole exhaust and 12 026 ppm 24 05 mgm 3 nitrogen dioxide and 99 18 ppm nitrogen oxides in filtered exhaust. The objective of this study was to determine percutaneous absorption of lead compounds including lead sulfate lead oxide lead powder and lead stearateThe lead content on the skin surface of 10 lead-battery workers was measured by the method of skin stripping and urinary lead content of rats was measured with epicutaneous application of four lead compounds.
 Source: shutterstock.com
Source: shutterstock.com
The objective of this study was to determine percutaneous absorption of lead compounds including lead sulfate lead oxide lead powder and lead stearateThe lead content on the skin surface of 10 lead-battery workers was measured by the method of skin stripping and urinary lead content of rats was measured with epicutaneous application of four lead compounds. Please enter positive values. Nitrogen N Molecular weight. Sources and pathways of exposure. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia carbon monoxide hydrogen hydrogen sulfide or methane.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
National Fire Protection Association. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Air - Prandtl Number - Prandtl number for air vs. National Fire Protection Association. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia carbon monoxide hydrogen hydrogen sulfide or methane.
 Source: youtube.com
Source: youtube.com
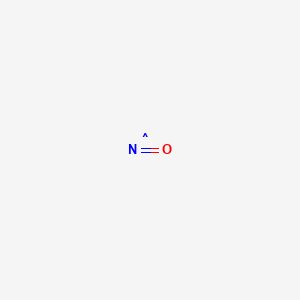
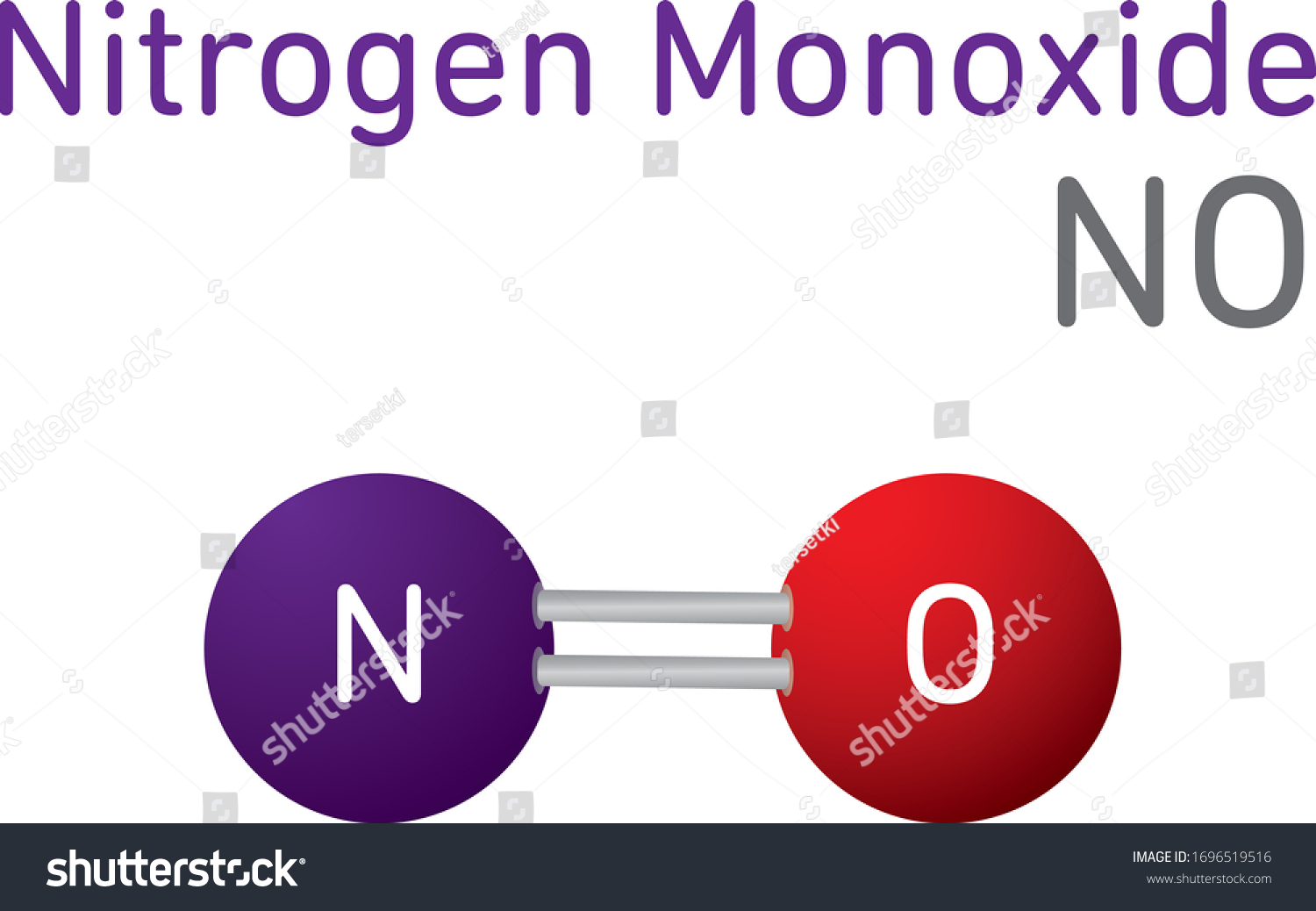
Please enter positive values. Key Takeaways Key Points. Please enter positive values. Air - Properties at Gas-Liquid Equilibrium Conditions - Properties of air change along the. Nitrogen oxides called NO x compounds are important for their explosive properties.
 Source: youtube.com
Source: youtube.com
Air - Properties at Gas-Liquid Equilibrium Conditions - Properties of air change along the. Levels of 15 03 ppm 3 06 SD mgm 3 nitrogen dioxide and 114 21 ppm nitrogen oxides were found in whole exhaust and 12 026 ppm 24 05 mgm 3 nitrogen dioxide and 99 18 ppm nitrogen oxides in filtered exhaust. Carbon monoxide - CO - 0 to trace ppm Ammonia. UHMWPE can be made through any catalyst technology although Ziegler catalysts are most common. Boiling Point BP Nitrogen changes its state from liquid to gas at -19579C -320422F or 7736K Nitrogen gas is a diamagnetic colorless odorless and tasteless gas.

S M M air where Sgas specific gravity Mgas molecular weight M air 2896443 gmole molecular weight of standard air - CRC 1983. Equations for Gas Specific Gravity and Molecular Weight Conversion. Carbon monoxide - CO - 0 to trace ppm Ammonia. Nitrogen N Molecular weight. At 760 mmHg and 20 C 1 ppm 1914 mgm 3 and 1 mgm 3 0523 ppm.
 Source: youtube.com
Source: youtube.com
Exposure to total diesel exhaust and filtered diesel exhaust significantly increased the number of animals with lung tumours adenomas and carcinomas to. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia carbon monoxide hydrogen hydrogen sulfide or methane. Temperature and Pressure - Online calculator figures and tables showing density and specific weight of nitrogen N 2 at temperatures ranging from -175 to 1325 C -280 to 2400 F at atmospheric and higher pressure - Imperial and SI Units. Carbon monoxide - CO - 0 to trace ppm Ammonia. S M M air where Sgas specific gravity Mgas molecular weight M air 2896443 gmole molecular weight of standard air - CRC 1983.
 Source: slideplayer.com
Source: slideplayer.com
Molecular diffusion often simply called diffusion is the thermal motion of all liquid or gas particles at temperatures above absolute zeroThe rate of this movement is a function of temperature viscosity of the fluid and the size mass of the particles. Carbon monoxide - CO - 0 to trace ppm Ammonia. Water is one of the most commonly found molecular compounds followed by carbon dioxide carbon monoxide hydrogen peroxide and methane. Its molecular weight is 4601 gmol melting point 112 C boiling point 2115 C and density 159 air 1. These properties are determined by the extremely strong and stable bond found in molecular diatomic nitrogen N 2 which has a bond dissociation energy of 945 kJmol 226 kcalmol.
 Source: slideplayer.com
Source: slideplayer.com
At 25 C 1 ppm 1882 mgm 3 and 1 mgm 3 0531 ppm. Water is one of the most commonly found molecular compounds followed by carbon dioxide carbon monoxide hydrogen peroxide and methane. The objective of this study was to determine percutaneous absorption of lead compounds including lead sulfate lead oxide lead powder and lead stearateThe lead content on the skin surface of 10 lead-battery workers was measured by the method of skin stripping and urinary lead content of rats was measured with epicutaneous application of four lead compounds. Read on to know more Read on to know more We all know that a compound is formed by combining two or more elements metal or. Nitrogen oxides called NO x compounds are important for their explosive properties.
 Source: en.wikipedia.org
Source: en.wikipedia.org
In ambient air the oxides of. Boiling Point BP Nitrogen changes its state from liquid to gas at -19579C -320422F or 7736K Nitrogen gas is a diamagnetic colorless odorless and tasteless gas. Water is one of the most commonly found molecular compounds followed by carbon dioxide carbon monoxide hydrogen peroxide and methane. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia carbon monoxide hydrogen hydrogen sulfide or methane. The high molecular weight makes it a very tough material but results in less efficient packing of the chains into the crystal structure as evidenced by densities of less than high-density polyethylene for example 09300935 gcm 3.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molecular weight of nitrogen monoxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.