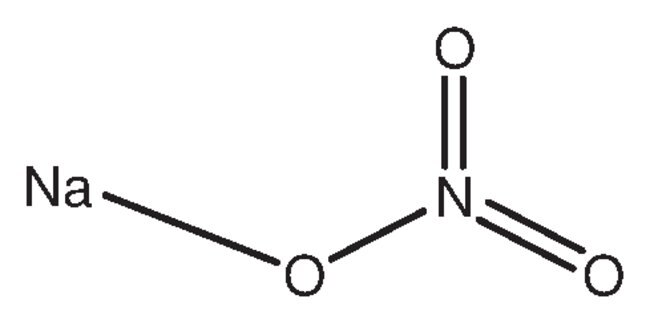
Molecular weight of sodium nitrate
Molecular Weight Of Sodium Nitrate. It is used in textile industries. Sodium nitrate 16 N 26 Na. 54 Likes 13 Comments - UCLA VA Physiatry Residency uclava_pmrresidency on Instagram. In some cases the majority of the weight of a compound arises from water.
 Molar Mass Molecular Weight Of Nano3 Sodium Nitrate Youtube From youtube.com
Molar Mass Molecular Weight Of Nano3 Sodium Nitrate Youtube From youtube.com
If large quantities are involved in fire or the combustible material is finely divided an explosion may result. It is used in textile industries. May explode under. Ozone O 3 207. This fertiliser is obtained from natural deposits in Chile and is usually marketed as moisture-resistant granules. The first study to show that nitrate supplementation may improve exercise efficiency was published in 2007 by Larsen et al.
It is used as a bleaching agent.
The oxidation potentials of various oxidants. 2 e-hydrogen peroxide H 2 O 2 178. 54 Likes 13 Comments - UCLA VA Physiatry Residency uclava_pmrresidency on Instagram. The oxidation potentials of various oxidants. It solves for total mass of a molecular formula average molecular weight. It is used in textile industries.
 Source: youtube.com
Source: youtube.com
May explode under. It is used in textile industries. Nitrate Reduces Oxygen Cost of Exercise and Improves Exercise Tolerance. Noncombustible but accelerates burning of combustible materials. Sodium Hypochlorite Structure Bleach.
 Source: brainly.in
Source: brainly.in
NaClO Uses Sodium hypochlorite It is used as a key ingredient in laundry bleach. Nitrate Reduces Oxygen Cost of Exercise and Improves Exercise Tolerance. Sodium reacts rapidly with water snow and ice to produce sodium hydroxide. May explode under. 2 e-hydrogen peroxide H 2 O 2 178.
 Source: findel-international.com
Source: findel-international.com
11 and atomic weight 229898. Calcium nitrate 155 N. This fertiliser is obtained from natural deposits in Chile and is usually marketed as moisture-resistant granules. The first study to show that nitrate supplementation may improve exercise efficiency was published in 2007 by Larsen et al. Sodium does not react with nitrogen even at very high temperatures but reacts with ammonia to form sodium amide.

It is used in textile industries. If large quantities are involved in fire or the combustible material is finely divided an explosion may result. Divide the molecular weight of the molecular formula by the the molecular weight of the empirical formula to find the ratio between the two. For large installations sodium chlorite chlorine gas Cl 2. Sodium Hypochlorite Structure Bleach.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Ozone O 3 207. Sodium reacts rapidly with water snow and ice to produce sodium hydroxide. Sodium Hypochlorite Structure Bleach. Latexfrac142286 gmol71143 gmol 2latex Since the weight of the molecular formula is twice the weight of the empirical formula there must be twice as many atoms but in the same. In some cases the majority of the weight of a compound arises from water.
 Source: clutchprep.com
Source: clutchprep.com
In that study nine well trained subjects consumed 01 mmolkg body mass BM per day of sodium nitrate or a placebo for 3 days before completing a continuous incremental cycle ergometer test. For large installations sodium chlorite chlorine gas Cl 2. Sodium Hypochlorite Structure Bleach. May explode under. It is used in textile industries.
 Source: youtube.com
Source: youtube.com
It is used in textile industries. The nitrogen is readily available and the sodium is of value to some market garden crops. Sodium does not react with nitrogen even at very high temperatures but reacts with ammonia to form sodium amide. Sodium reacts rapidly with water snow and ice to produce sodium hydroxide. Ozone O 3 207.

The first study to show that nitrate supplementation may improve exercise efficiency was published in 2007 by Larsen et al. Glaubers salt Na 2 SO 4 H 2 O 10 is a white crystalline solid with greater than 50 water by weight. Its a soft metal reactive and with a low melting point with a relative density of 097 at 20ºC 68ºF. Noncombustible but accelerates burning of combustible materials. Molecular Weight Molar Mass.
 Source: youtube.com
Source: youtube.com
When metallic sodium is exposed to air it loses its silver appearance and develops an opaque grey colour layer which is a coating of sodium oxide. It is expensive and is not widely used. It is used as an oxidizing. In some cases the majority of the weight of a compound arises from water. For large installations sodium chlorite chlorine gas Cl 2.
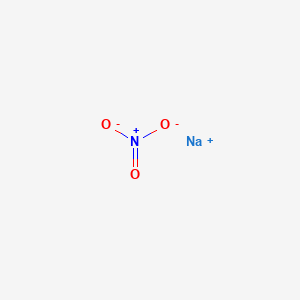
If large quantities are involved in fire or the combustible material is finely divided an explosion may result. It is used as an oxidizing. This is a double salt of calcium nitrate and ammonium nitrate in prilled form. In some cases the majority of the weight of a compound arises from water. Calcium nitrate 155 N.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molecular weight of sodium nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.